Label: DUKAL STING RELIEF PAD- benzocaine swab
- NDC Code(s): 65517-0005-1
- Packager: Dukal LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- INDICATIONS & USAGE
- Directions
- WARNINGS
- Inactive Ingredient
-
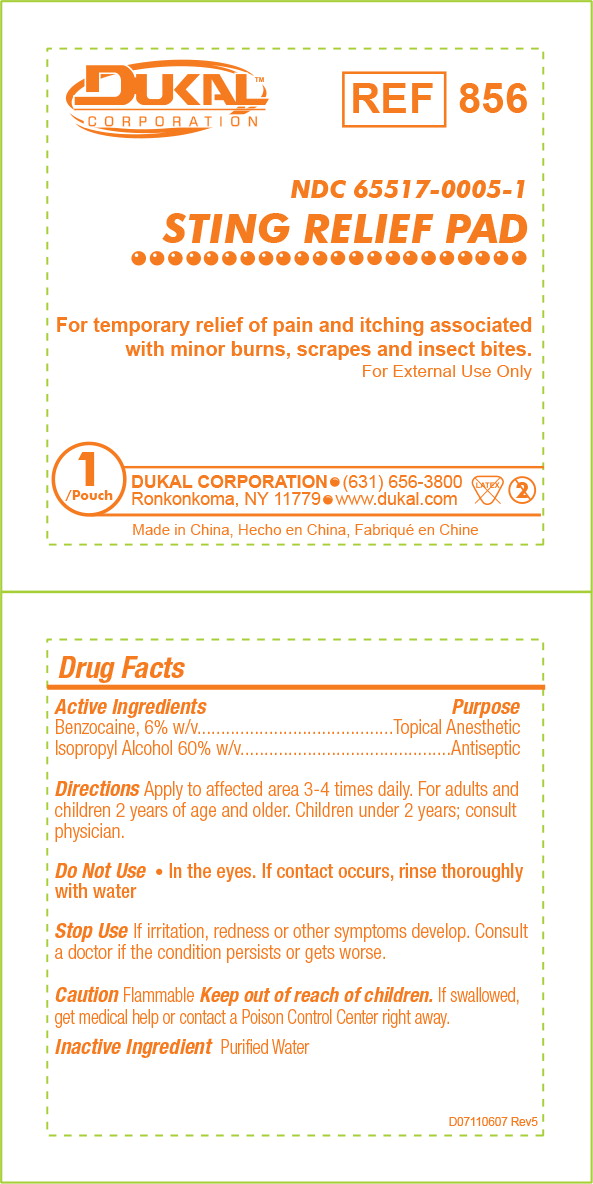
Principal Display Panel - 0.4 mL Pouch Label
DUKAL™
CORPORATIONREF
856NDC 65517-0005-1
STING RELIEF PADFor temporary relief of pain and itching associated with minor burns, scrapes and insect bites.
For External Use Only
1 Pouch
DUKAL CORPORATION • (631) 656-3800
Ronkonkoma, New York • www.dukal.comMade in China, Hecho en China, Fabriqué en Chine
-
INGREDIENTS AND APPEARANCE
DUKAL STING RELIEF PAD
benzocaine swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65517-0005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 60 mg in 1 mL ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.6 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-0005-1 0.4 mL in 1 POUCH; Type 0: Not a Combination Product 06/01/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/01/2010 Labeler - Dukal LLC (791014871)