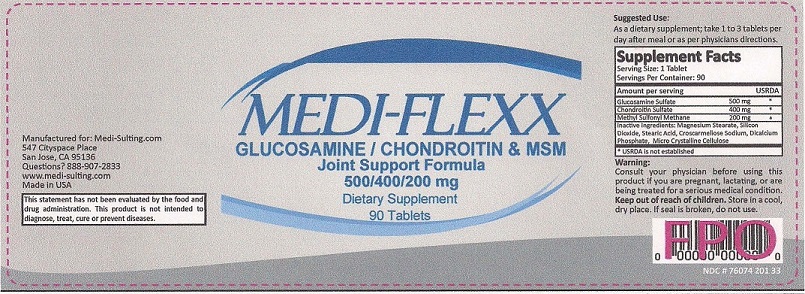
MEDI-FLEXX JOINT SUPPORT FORMULA- glucosamine sulfate, chondroitin sulfate (chicken) and dimethyl sulfone tablet
TWO HIP CONSULTING, LLC
----------
WARNING:
CONSULT YOUR PHYSICIAN BEFORE USING THIS PRODUCT IF YOU ARE PREGNANT, LACTATING, OR ARE BEING TREATED FOR A SERIOUS MEDICAL CONDITION. KEEP OUT OF REACH OF CHILDREN.
| MEDI-FLEXX
JOINT SUPPORT FORMULA
glucosamine sulfate chondroitin sulfate (bovine) dimethyl sulfone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - TWO HIP CONSULTING, LLC (965352896) |
Revised: 4/2016
Document Id: 8f60eecd-95e1-41f2-9beb-ab1067e830d9
Set id: 67916a8f-5000-4b2b-bf8c-47d2e014176b
Version: 3
Effective Time: 20160411
TWO HIP CONSULTING, LLC