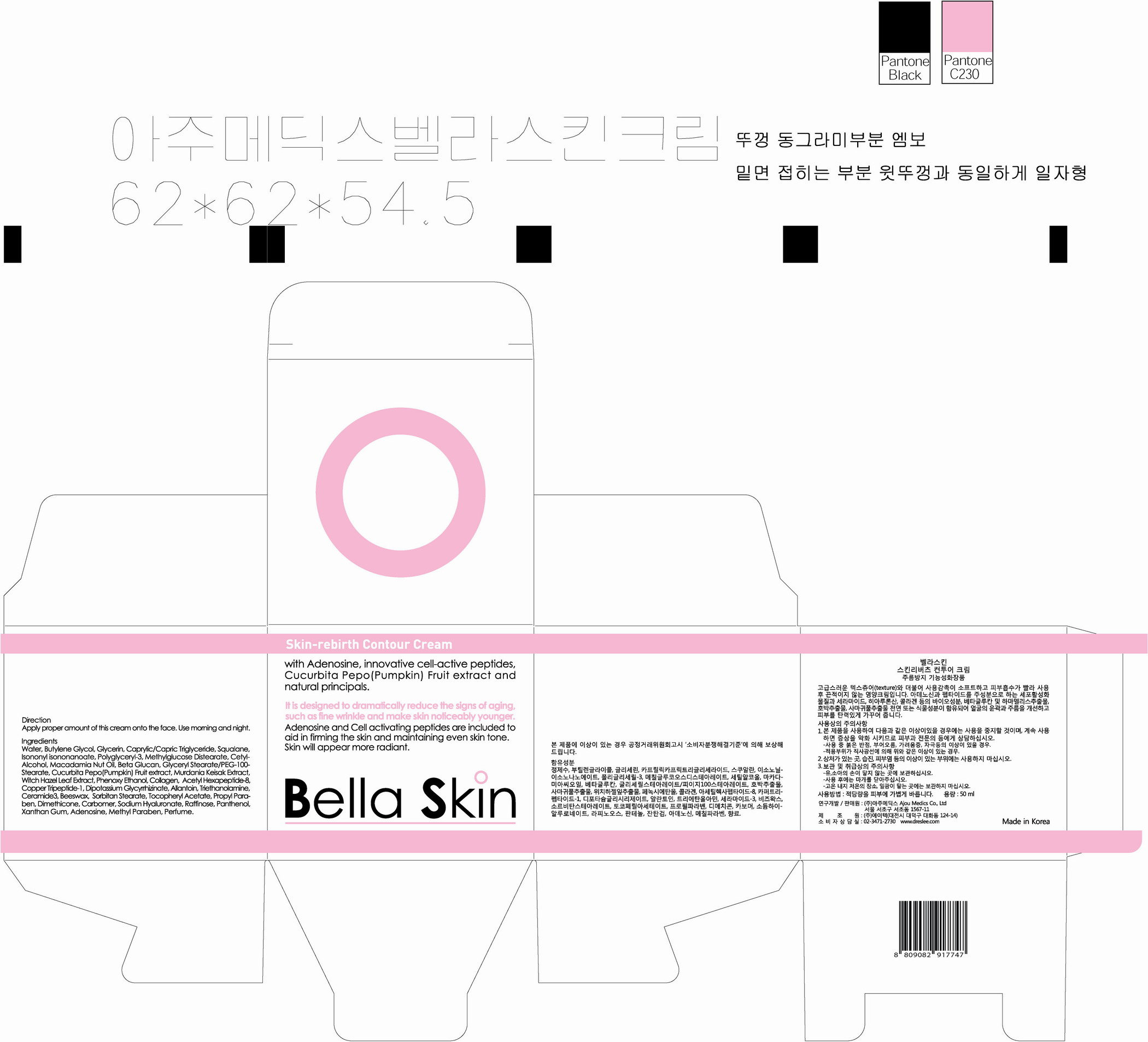
active ingredient: adenosine
inactive ingredient: Water, Butylene Glycol, Glycerin, Caprylic/Capric Triglyceride, Isononyl Isononanoate, Squalane, Polyglyceryl 3-Methylglucose Distearate, Cetanol, Macadamia Integrifolia Seed Oil, Beta-Glucan, Glyceryl Stearate / PEG-100 Stearate,
Cucubita Pepo(Pumpkin) Fruit Extract, Murdania Keisak Extract, Hamamelis Virginiana(Witchhazel) Leaf Extract, Beeswax, Sorbitan Stearate, Collagen, Dimethicone, Raffinose, Triethanolamine, Carbomer, Methyl Paraben, Phenoxy Ethanol, Fragrance, Allantoin, Ceramide3, Propyl Paraben, Dipotassium Glycyrrhizate, Panthenol, Xanthan Gum, Tocopheryl Acetate, Sodium Hyaluronate, Copper Tripeptide-1, Acetyl Hexapeptide-8
keep out of reach of the children
Apply Proper Amount of the cream on the face.
■ For external use only.
■ Avoid contact with eyes.
■ Do not swallow. If swallowed, get medical help.
■ Keep out of reach of children.
■ Stop use and ask doctor if rash and irritation develops.
wash the skin to be applied