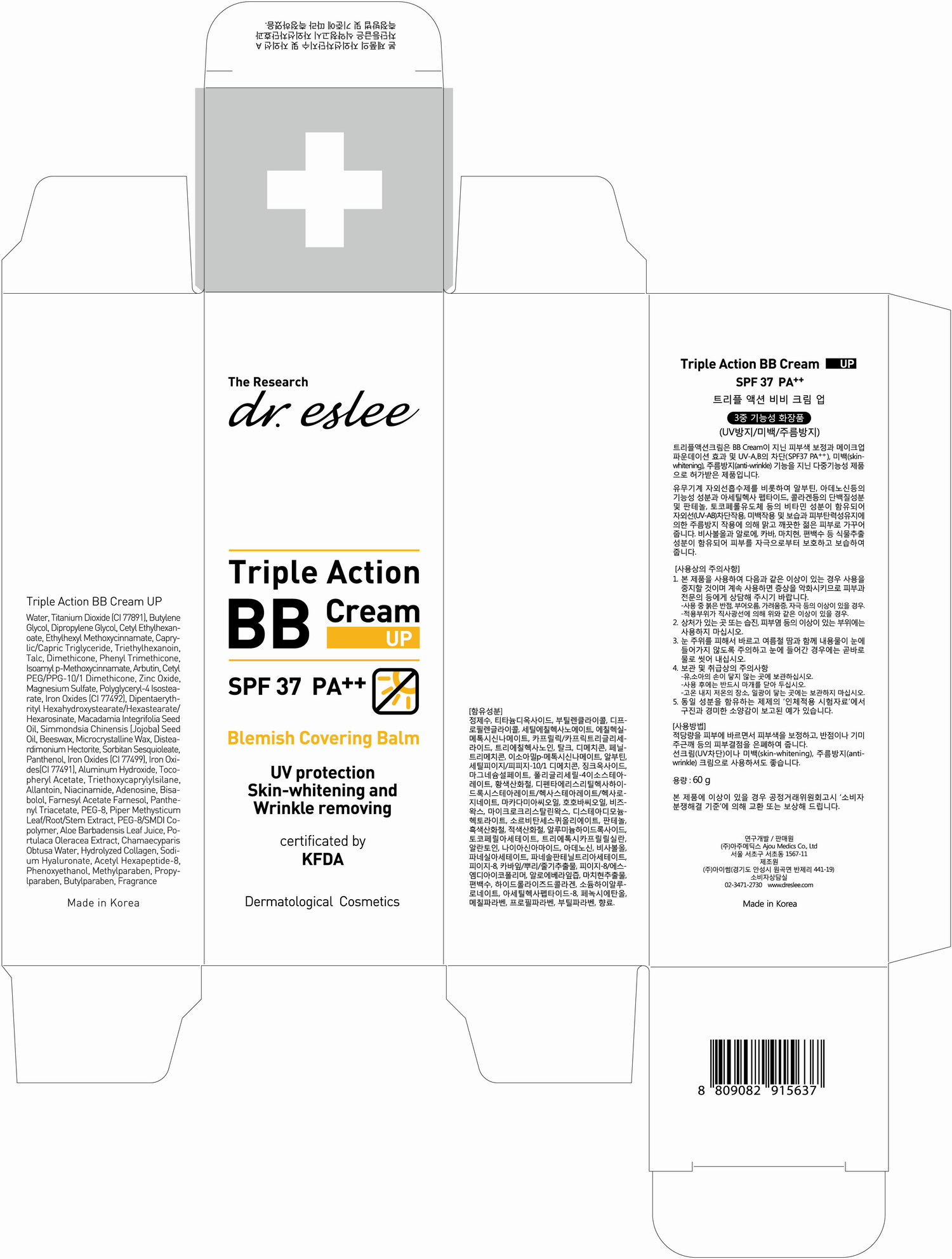
active ingredient: adenosine
inactive ingredient: Water, Butylene Glycol, Dipropylene Glycol, Cetyl Ethylhexanoate, Ethylhexyl Methoxycinnamate, Caprylic/Capric Triglyceride, Triethylhexanoin, Talc, Dimethicone, Phenyl Trimethicone, Isoamyl p-Methoxycinnamate, Arbutin, Cetyl PEG/PPG-10/1 Dimethicone, Zinc Oxide, Magnesium Sulfate, Polyglyceryl-4 Isostearate, Iron Oxides (CI 77492), Dipentaerythrityl Hexahydroxystearate / Hexastearate / Hexarosinate, Macadamia Integrifolia Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Beeswax, Microcrystalline Wax, Disteardimonium Hectorite, Sorbitan Sesquioleate, Panthenol, Iron Oxides (CI 77499), Iron Oxides (CI 77491), Aluminum Hydroxide, Tocopheryl Acetate, Triethoxycaprylylsilane, Allantoin, Bisabolol, Farnesyl Acetate, Farnesol , Panthenyl Triacetate, PEG-8, Piper Methysticum Leaf/Root/Stem Extract, PEG-, /SMDI Copolymer, Aloe Barbadensis Leaf Juice, Portulaca Oleracea Extract, Chamaecyparis Obtusa Water, Hydrolyzed Collagen, Sodium Hyaluronate, Acetyl Hexapeptide-8, , Phenoxyethanol, Methylparaben, Propylparaben , Butylparaben, Fragrance
Helps to protect from UV-Rays, skin whitening and wrinkle removing
keep out of reach of the children
Apply Proper Amount of the cream on skin.
■ For external use only.
■ Avoid contact with eyes.
■ Do not swallow. If swallowed, get medical help.
■ Keep out of reach of children.
■ Stop use and ask doctor if rash and irritation develops.
wash the skin to be applied