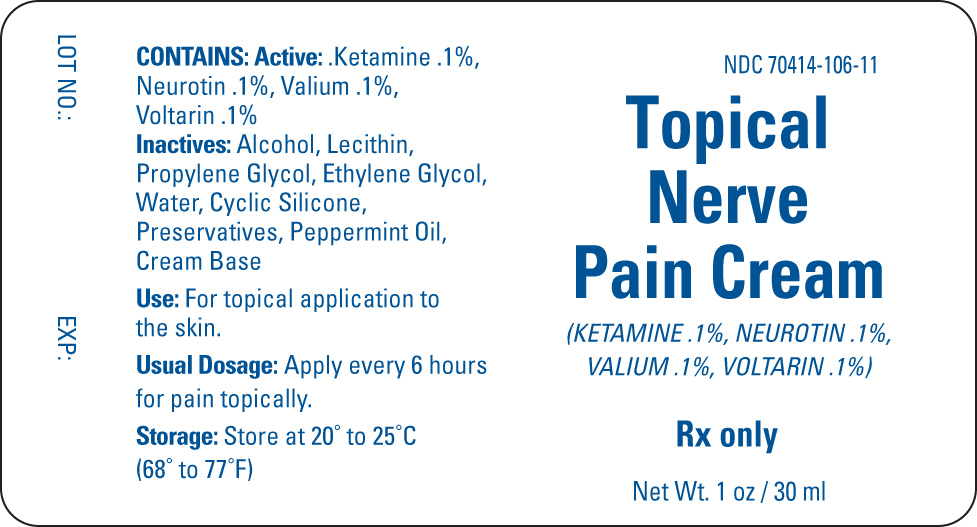
TOPICAL NERVE PAIN- ketamine, neurotin, valium, voltarin cream
DR MARC'S MANUFACTURING AND SALES
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
| TOPICAL NERVE PAIN
ketamine, neurotin, valium, voltarin cream |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - DR MARC'S MANUFACTURING AND SALES (084006446) |
Revised: 4/2018
Document Id: d414849f-c26b-46a0-bd39-6c767ccdbee8
Set id: 66be7f5b-0280-41ca-8828-731bba42f025
Version: 2
Effective Time: 20180417
DR MARC'S MANUFACTURING AND SALES