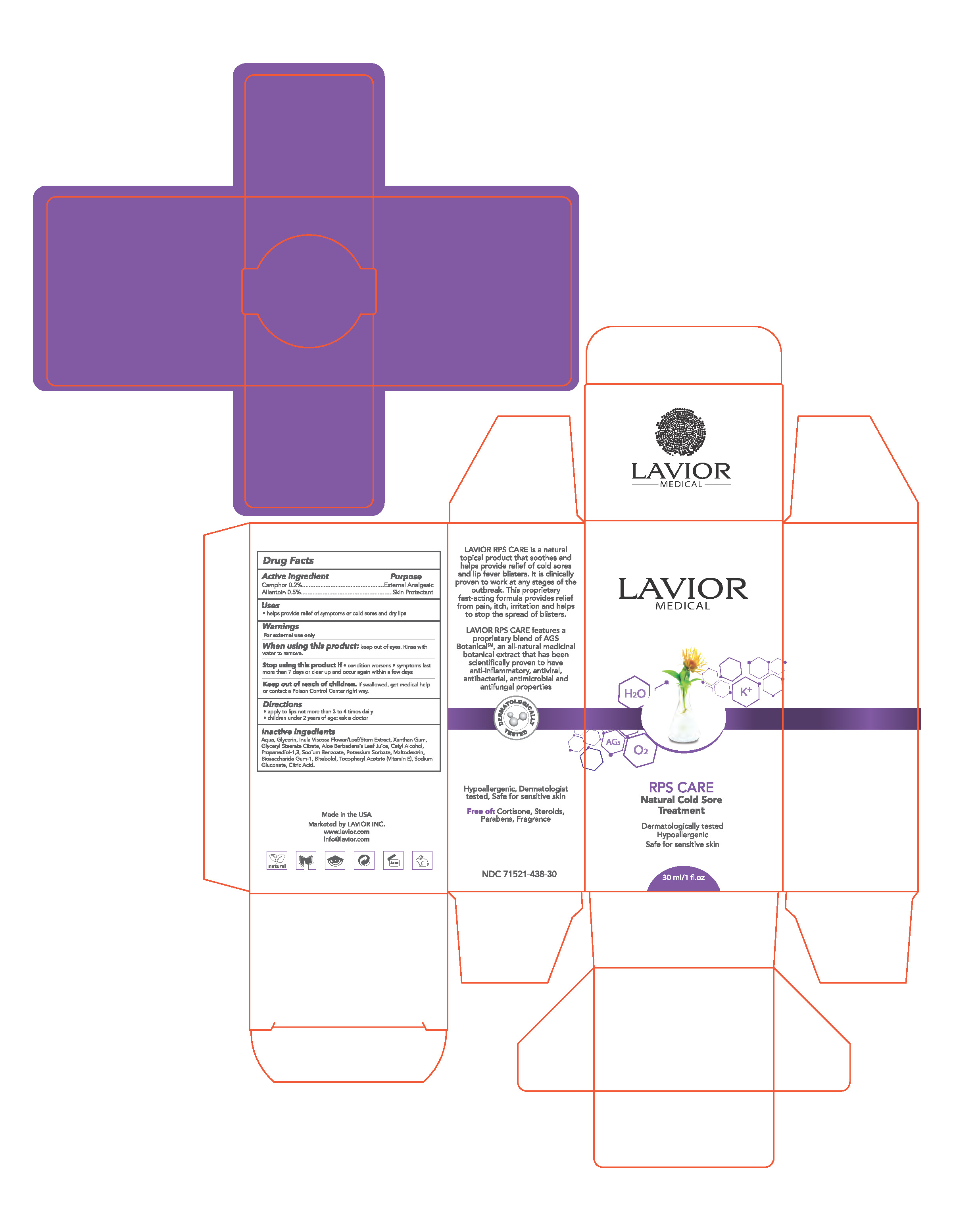
RPS CARE- allantoin, camphor cream
Lavior Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Directions
- apply to lips not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Aqua, Glycerin, Inula Viscosa Flower/Leaf/Stem Extract, Xanthan Gum, Glyceryl Stearate Citrate, Aloe Barbadensis Leaf Juice, Cetyl Alcohol, Propanediol-1,3, Sodium Benzoate, Potassium Sorbate, Maltodextrin, Biosaccharide Gum-1, Bisabolol, Tocopheryl Acetate (Vitamin E), Sodium Gluconate, Citric Acid.
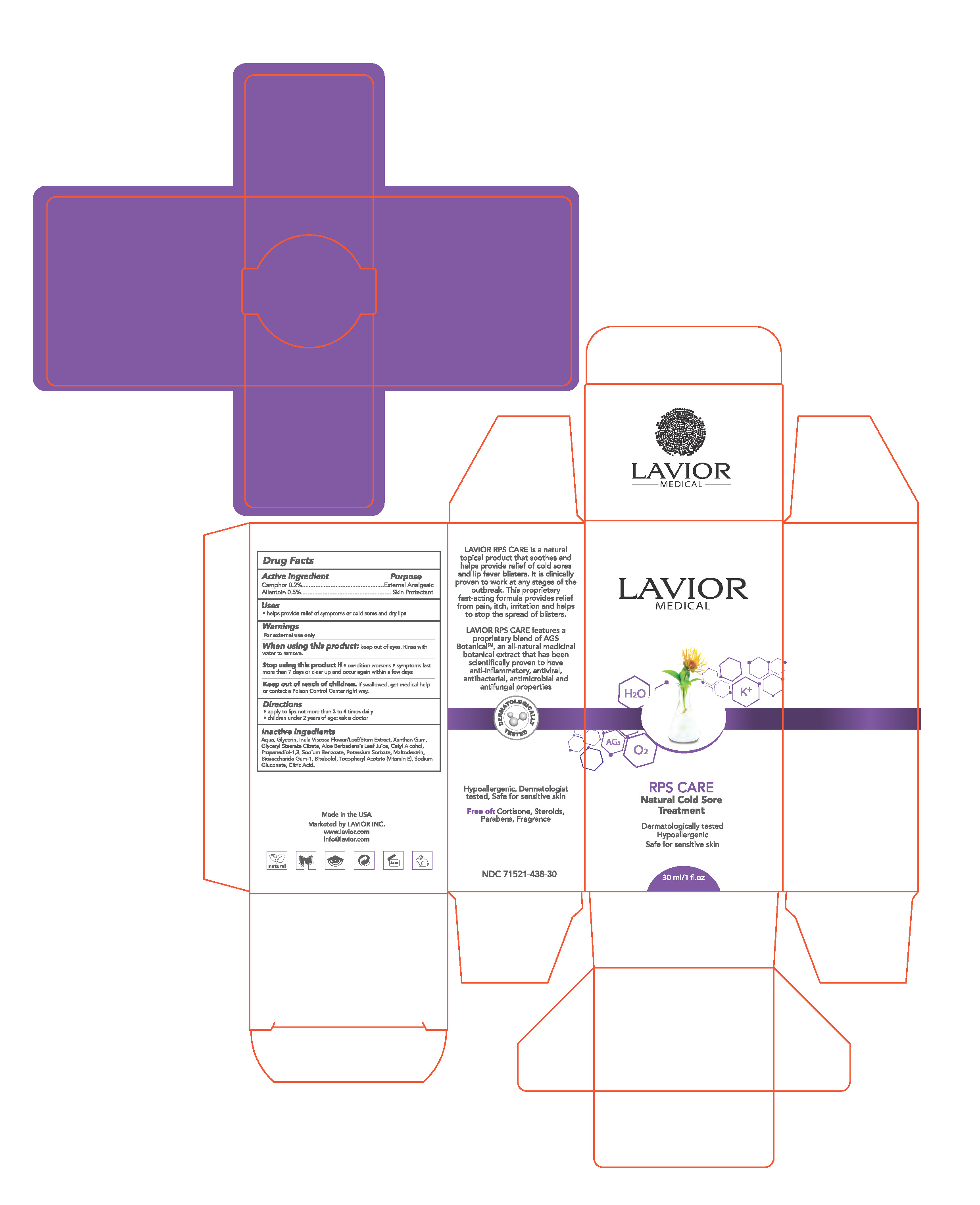
| RPS CARE
allantoin, camphor cream |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Lavior Inc. (080685327) |
Revised: 6/2021
Document Id: c3f88f60-08e1-352a-e053-2995a90a4b02
Set id: 6639c214-4b93-ddb5-e053-2a91aa0aedba
Version: 3
Effective Time: 20210604
Lavior Inc.