Label: REFILL BUNDLE 7 LARGE OFFICE- benzalkonium chloride kit
- NDC Code(s): 49687-0011-1, 49687-0029-0
- Packager: CMC Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
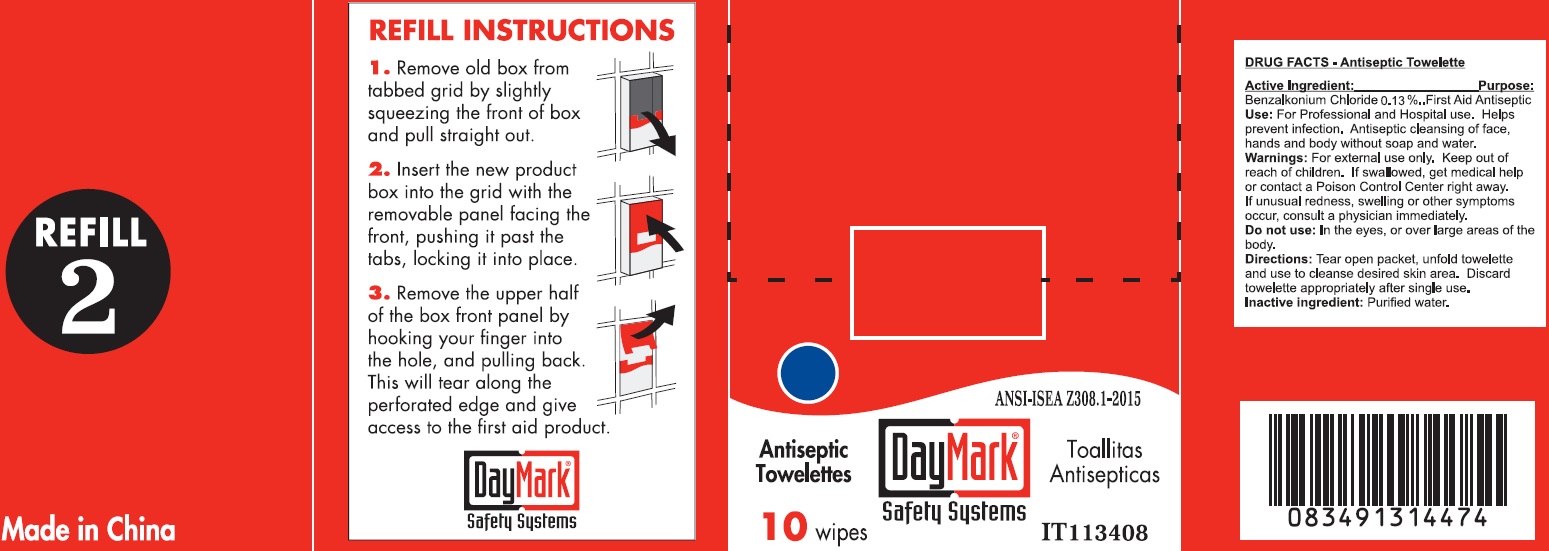
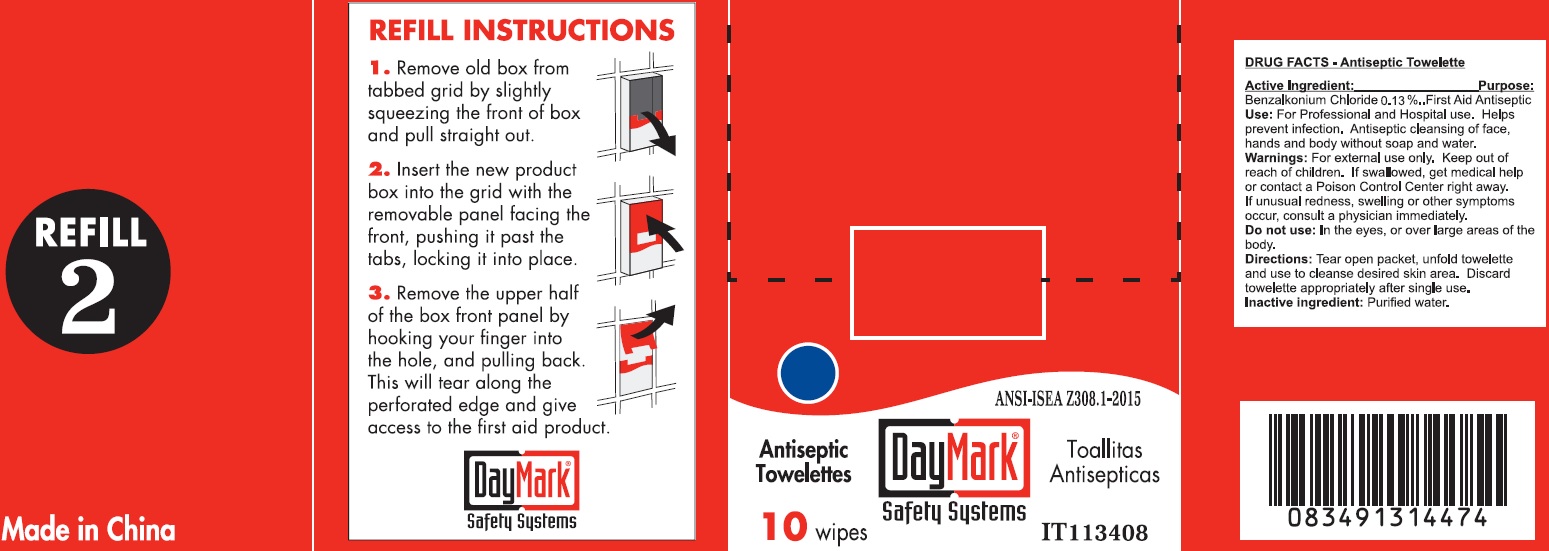
- Antiseptic Towelette 10 count 49687-0011-0 DRUG FACTS
- Active Ingredient:
- Use:
- Warnings:
- Directions:
- Inactive ingredient:
- Package Labeling:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
REFILL BUNDLE 7 LARGE OFFICE
benzalkonium chloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49687-0029 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49687-0029-0 1 in 1 KIT 02/13/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 PATCH 9 g Part 1 of 1 ANTISEPTIC TOWELETTES
benzalkonium chloride clothProduct Information Item Code (Source) NDC:49687-0011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49687-0011-1 10 in 1 BOX 1 0.9 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 02/13/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/13/2018 Labeler - CMC Group, Inc. (117201448)