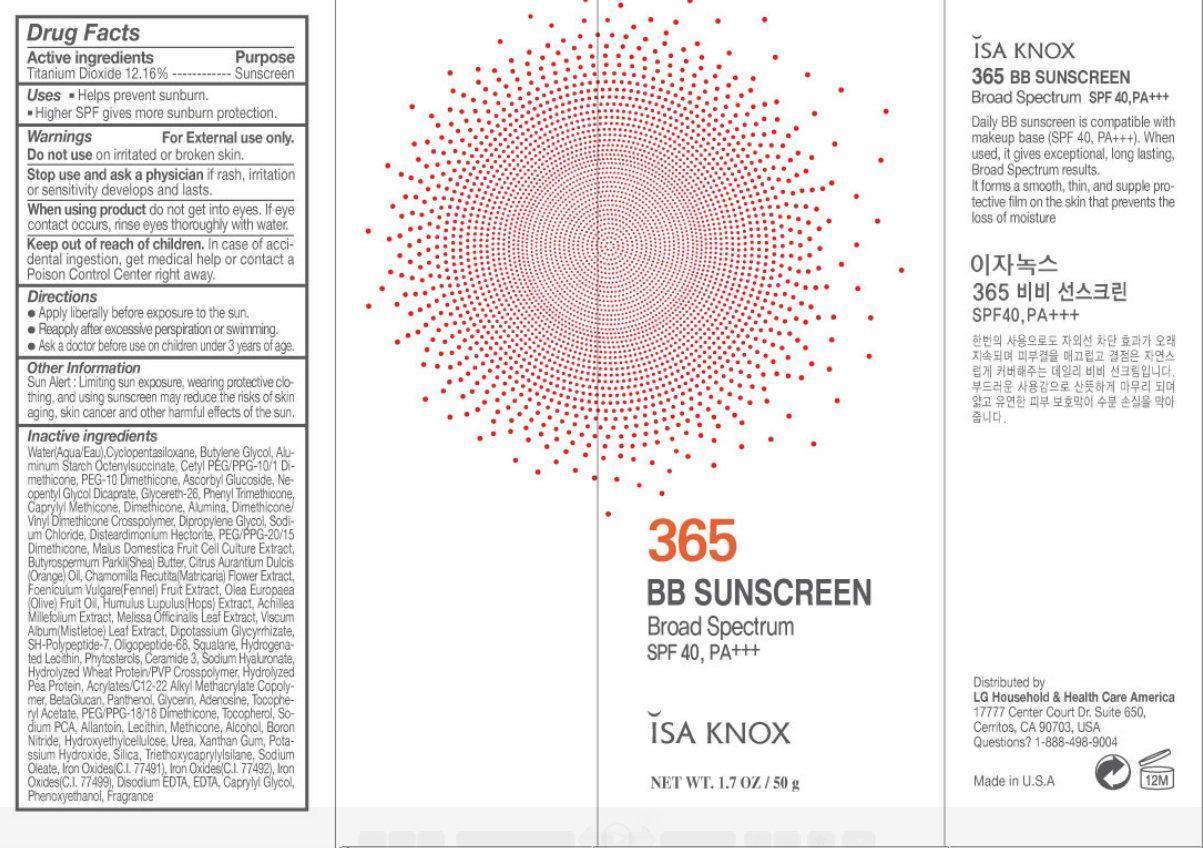
Label: ISA KNOX 365 BB SUNSCREEN BROAD SPECTRUM SPF 40, PA- titanium dioxide cream
- NDC Code(s): 53208-000-00
- Packager: LG Household and Healthcare, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Water(Aqua/Eau), Cyclopentasiloxane, Butylene Glycol, Aluminum Starch Octenylsuccinate, Cetyl PEG/PPG-10/1 Dimethicone, PEG-10 Dimethicone, Ascorbyl Glucoside, Neopentyl Glycol Dicaprate, Glycereth-26, Phenyl Trimethicone, Caprylyl Methicone, Dimethicone, Alumina, Dimethicone Vinyl Dimethicone Crosspolymer, Dipropylene Glycol, Sodium Chloride, Disteardimonium Hectorite, PEG/PPG-20/15 Dimethicone, Malus Domestica Fruit Cell Culture Extract, Butyrospermum Parkii (Shea) Butter, Citrus Aurantium Dulcis (Orange) Oil, Chamomilla Recutita (Matricaria) Flower Extract, Foeniculum Vulgare (Fennel) Fruit Extract, Olea Europaea (Olive) Fruit Oil, Humulus Lupulus (Hops) Extract, Achillea Millefolium Extract, Melissa Officinalis Leaf Extract, Viscum Album (Mistletoe) Leaf Extract, Dipotassium Glycyrrhizate, SH-Polypeptide-7, Oligopeptide-68, Squalane, Hydrogenated Lecithin, Phytosterols, Ceramide 3, Sodium Hyaluronate, Hydrolyzed Wheat Protein/PVP Crosspolymer, Hydrolyzed Pea Protein, Acrylates/C12-22 Alkyl Methacrylate Copolymer, BetaGlucan, Panthenol, Glycerin, Adenosine, Tocopheryl Acetate, PEG/PPG-18/18 Dimethicone, Tocopherol, Sodium PCA, Allantoin, Lecithin, Methicone, Alcohol, Boron Nitride, Hydroxyethylcellulose, Urea, Xanthan Gum, Potassium Hydroxide, Silica, Triethoxycaprylylsilane, Sodium Oleate, Iron Oxides (C.I. 77491), Iron Oxides (C.I. 77492), Iron Oxides (C.I. 77499), Disodium EDTA, EDTA, Caprylyl Glycol, Phenoxyethanol, Fragrance.
-
ISA KNOX 365 BB SUNSCREEN Broad Spectrum SPF 40, PA
Daily BB sunscreen is compatible with makeup base (SPF 40, PA+++). When used, it gives exceptional, long lasting, Broad Spectrum results. It forms a smooth, thin, and supple protective film on the skin that prevents the loss of moisture Distributed by 17777 Center Court Dr. Suite 650, Cerritos, CA 90703, USA Questions? 1-888-498-9004 Made in U.S.A.
LG Household and Health Care America - PRINCIPAL DISPLAY PANEL
- Product Label
-
INGREDIENTS AND APPEARANCE
ISA KNOX 365 BB SUNSCREEN BROAD SPECTRUM SPF 40, PA
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53208-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 121.6 mg in 1 g Inactive Ingredients Ingredient Name Strength ORANGE OIL (UNII: AKN3KSD11B) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) NEOPENTYL GLYCOL DICAPRATE (UNII: 77T908SE82) GLYCERETH-26 (UNII: NNE56F2N14) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) DIMETHICONE (UNII: 92RU3N3Y1O) ALUMINUM OXIDE (UNII: LMI26O6933) DIPROPYLENE GLYCOL (UNII: E107L85C40) SODIUM CHLORIDE (UNII: 451W47IQ8X) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PEG/PPG-20/15 DIMETHICONE (UNII: 06R6X77P9C) SHEA BUTTER (UNII: K49155WL9Y) CHAMOMILE (UNII: FGL3685T2X) FENNEL SEED (UNII: G3QC02NIE6) OLIVE OIL (UNII: 6UYK2W1W1E) HOPS (UNII: 01G73H6H83) ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) MELISSA OFFICINALIS LEAF (UNII: 50D2ZE9219) VISCUM ALBUM FRUITING TOP (UNII: BK9092J5MP) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) SQUALANE (UNII: GW89575KF9) CERAMIDE 3 (UNII: 4370DF050B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PANTHENOL (UNII: WV9CM0O67Z) GLYCERIN (UNII: PDC6A3C0OX) ADENOSINE (UNII: K72T3FS567) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) ALLANTOIN (UNII: 344S277G0Z) ALCOHOL (UNII: 3K9958V90M) BORON NITRIDE (UNII: 2U4T60A6YD) UREA (UNII: 8W8T17847W) XANTHAN GUM (UNII: TTV12P4NEE) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM OLEATE (UNII: 399SL044HN) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) EDETATE DISODIUM (UNII: 7FLD91C86K) EDETIC ACID (UNII: 9G34HU7RV0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53208-000-00 1 in 1 PACKAGE 12/19/2017 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/13/2013 Labeler - LG Household and Healthcare, Inc. (688276187) Establishment Name Address ID/FEI Business Operations Englewood Lab, Inc. 172198223 manufacture(53208-000)