Label: TUKOL A- dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride liquid
- NDC Code(s): 50066-536-24
- Packager: Genomma Lab USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 28, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
Do not use in a child
who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if the child has
- Ask a doctor or pharmacist before use if the child is
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
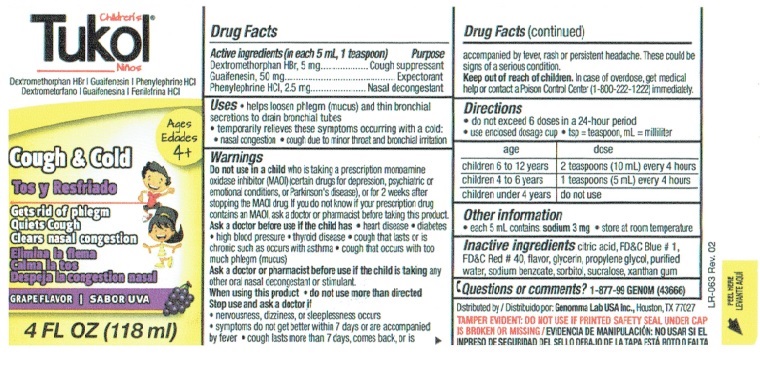
Children's Tukol Bottle Label
Children’s Tukol®
Cough Suppressant/Expectorant/Nasal Decongestant
Dextromethorphan HBr | Guaifenesin | Phenylephrine HCIAges 4+
Cough and Cold
Gets rid of phlegm
Quiets Cough
Clears nasal congestionGrape Flavor
4 FL OZ (118 ml)
Drug Facts
Active ingredients Purpose
(in each 5 mL, 1 teaspoon)
Dextromethorphan HBr, 5 mg ...............Cough suppressant
Guaifenesin, 50 mg. .......................................Expectorant
Phenylephrine HCI, 2.5 mg ................... Nasal decongestantUses
• helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
• temporarily relieves these symptoms occurring with a cold:
• nasal congestion
• cough due to minor throat and bronchial irritationWarnings
Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if the child has
• heart disease
• diabetes
• high blood pressure
• thyroid disease
• cough that lasts or is chronic such as occurs with asthma
• cough that occurs with too much phlegm (mucus)Ask a doctor or pharmacist before use if the child is taking any other oral nasal decongestant or stimulant.
When using this product
• do not use more than directedStop use and ask a doctor if
• nervousness, dizziness, or sleeplessness occurs
• symptoms do not get better within 7 days or are accompanied by fever
• cough last more than 7 days, comes back, or is accompanied by fever, rash or persistent headache. These could be signs of a serious condition.Drug Facts (continued)
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) immediately.
Directions
• do not exceed 6 doses in a 24-hour period
• use enclosed dosage cup
• tsp = teaspoon, mL = milliliterage dose
children 6 to 12 years 2 teaspoons (10 mL) every 4 hours
children 4 to 6 years 1 teaspoons (5 mL) every 4 hours
children under 4 years do not useOther information
• each 5 mL contains: sodium 3 mg
• store at room temperatureInactive ingredients citric acid, FD&C Blue # 1, FD&C Red # 40, flavor, glycerin, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, xanthan gum
Questions or comments? 1-877-99 GENOM (43666)
Distributed by Genomma Lab USA., Houston, TX, 77027
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
LR-063 Rev. 2
PEEL HERE
-
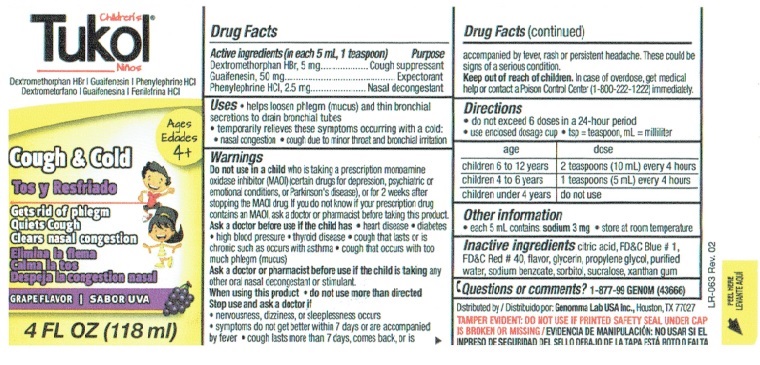
Children's Tukol Box Label
Children’s Tukol®
Cough Suppressant/Expectorant/Nasal Decongestant
Dextromethorphan HBr | Guaifenesin | Phenylephrine HCIDO NOT USE IF PRINTED SEAL UNDER CAP
IS TORN OR MISSINGChildren’s Tukol®
Cough Suppressant/Expectorant/Nasal Decongestant
Dextromethorphan HBr | Guaifenesin | Phenylephrine HCI
Ages 4+Cough and Cold
Gets rid of phlegm
Quiets Cough
Clears nasal congestionGrape Flavor
4 FL OZ (118 ml)
LOT No.
Exp.Drug Facts
Active ingredients Purpose
(in each 5 mL, 1 teaspoon)
Dextromethorphan HBr, 5 mg ...............Cough suppressant
Guaifenesin, 50 mg. .......................................Expectorant
Phenylephrine HCI, 2.5 mg ................... Nasal decongestantUses
• helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
• temporarily relieves these symptoms occurring with a cold:
• nasal congestion
• cough due to minor throat and bronchial irritationWarnings
Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if the child has
• heart disease
• diabetes
• high blood pressure
• thyroid disease
• cough that lasts or is chronic such as occurs with asthma
• cough that occurs with too much phlegm (mucus)Ask a doctor or pharmacist before use if the child is taking any other oral nasal decongestant or stimulant.
When using this product
• do not use more than directedStop use and ask a doctor if
• nervousness, dizziness, or sleeplessness occurs
• symptoms do not get better within 7 days or are accompanied by fever
• cough last more than 7 days, comes back, or is accompanied by fever, rash or persistent headache. These could be signs of a serious condition.Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) immediately.
Distributed by
Genomma Lab USA Inc.,
Houston, TX 77027
Genomma Lab.®3
66975-3
8/17Drug Facts (continued)
Directions
• do not exceed 6 doses in a 24-hour period
• use enclosed dosage cup
• tsp = teaspoon, mL = milliliterage dose
children 6 to 12 years 2 teaspoons (10 mL) every 4 hours
children 4 to 6 years 1 teaspoons (5 mL) every 4 hours
children under 4 years do not useOther information
• each 5 mL contains: sodium 3 mg
• store at room temperatureInactive ingredients citric acid, FD&C Blue # 1, FD&C Red # 40, flavor, glycerin, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, xanthan gum
Questions or comments? 1-877-99 GENOM (43666)
BX-037 Rev. 02
2FTAP1MUEWW1B
-
INGREDIENTS AND APPEARANCE
TUKOL A
dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50066-536 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 5 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 50 mg in 5 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor GRAPE (GRAPE FLAVOR) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50066-536-24 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/09/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/09/2014 Labeler - Genomma Lab USA, Inc. (832323534) Registrant - AptaPharma Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(50066-536)