STOOL SOFTENER- docusate sodium capsule
Good Sense (Geiss, Destin & Dunn, Inc.)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
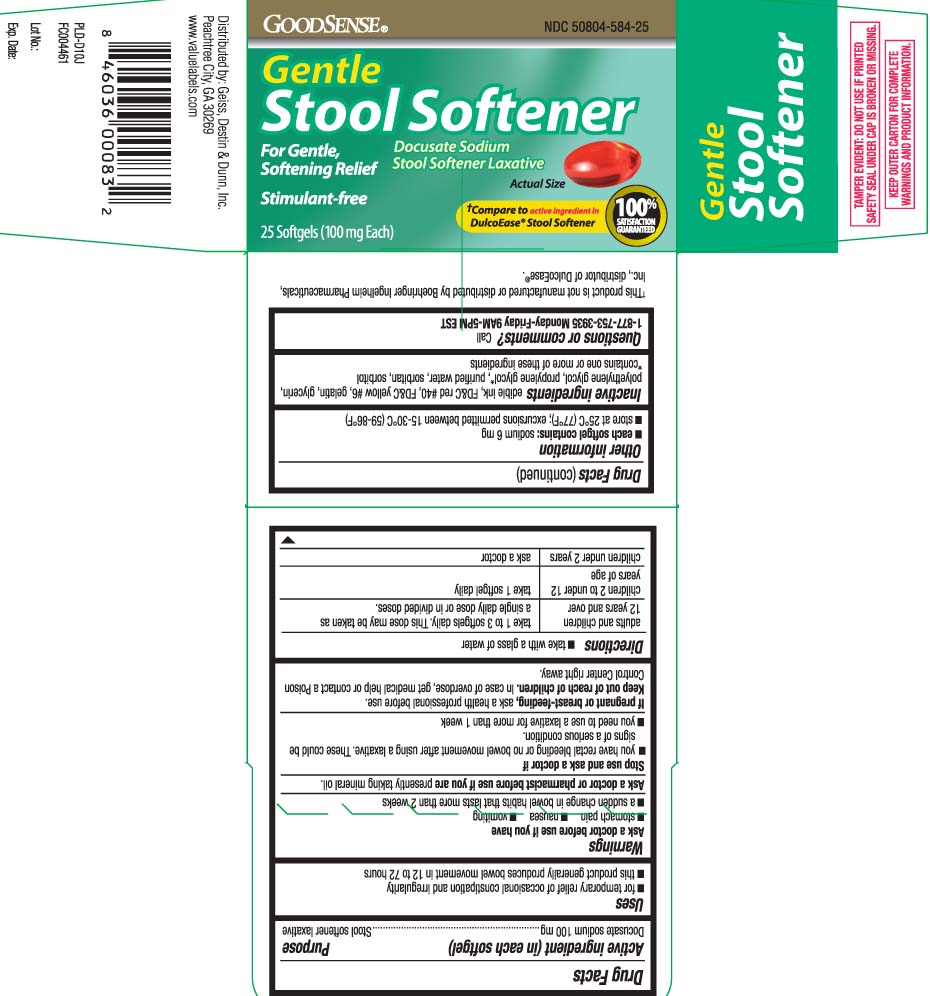
Uses
- for temporary relief of occasional constipation and irregularity
- this product generally produces a bowel movement within 12 to 72 hours
Warnings
Ask a doctor before use if
- stomach pain
- nausea
- vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
Directions
- take with a glass of water
| adults and children 12 over | take 1 to 3 softgels daily. This dose may be taken as a single daily dose or in divided doses. |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years | ask a doctor |
Other information
- each softgel contains: sodium 6 mg
- store 25ºC (77ºF); excursion permitted between 15-30ºC (59-86ºF)
Inactive ingredients
edible ink, FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol*, purified water and sorbitan, sorbitol
*contains one or more of these ingredients
Principal Display Panel
Gentle Stool Softener
Docusate Sodium
Stool Softener Laxative
For Gentle, Softening Relief
Stimulant-Free
†Compare to active ingredient in DulcoEase® Stool Softener
†This product is not manufactured or distributed by Boehringer Ingelheim Pharmaceuticals, distributor of DulcoEase®.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by: Geiss, Destin & Dunn, Inc.
Peachtree City, GA 30269
| STOOL SOFTENER
docusate sodium capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Good Sense (Geiss, Destin & Dunn, Inc.) (076059836) |