Label: KINDEST KARE AIR-INFUSED FOAM ANTISEPTIC HANDRUB- alcohol liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 11084-809-13, 11084-809-41 - Packager: SC Johnson Professional USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 21, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Questions or comments?
-
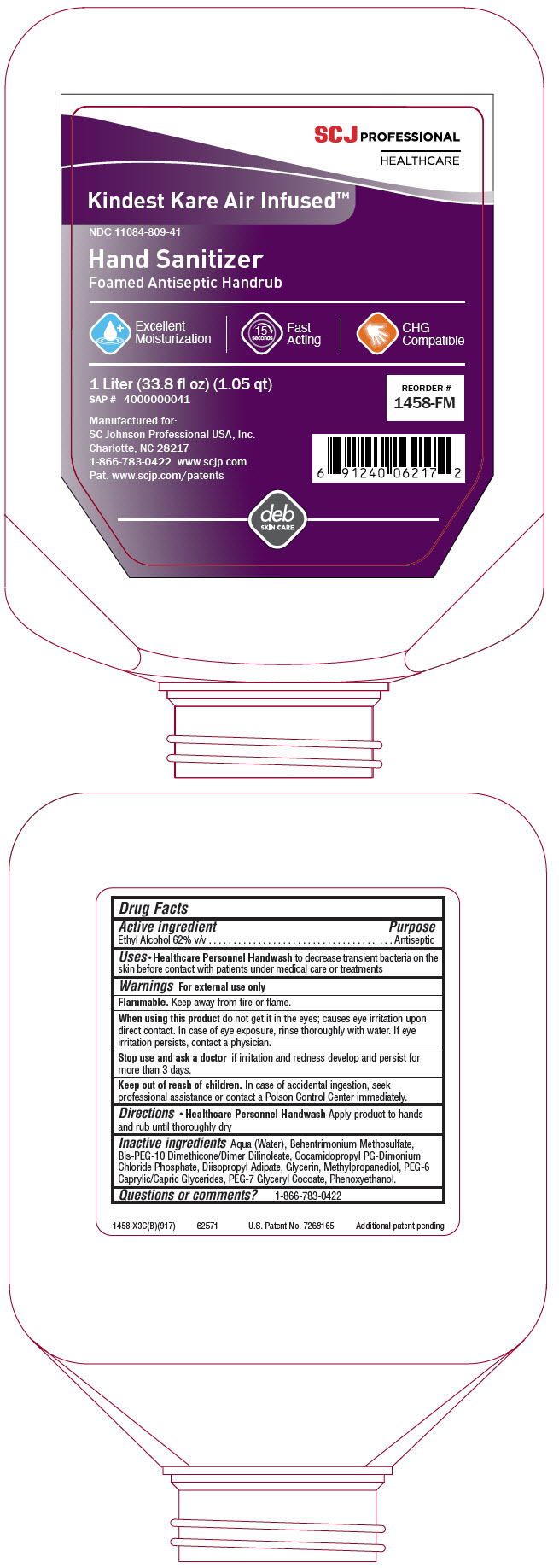
PRINCIPAL DISPLAY PANEL - 1 Liter Bottle Label
SCJ PROFESSIONAL
HEALTHCAREKindest Kare Air Infused™
NDC 11084-809-41
Hand Sanitizer
Foamed Antiseptic HandrubExcellent
Moisturization15
seconds
Fast
ActingCHG
Compatible1 Liter (33.8 fl oz) (1.05 qt)
SAP # 4000000041REORDER #
1458-FMManufactured for:
SC Johnson Professional USA, Inc.
Charlotte, NC 28217
1-866-783-0422 www.scjp.com
Pat. www.scjp.com/patentsdeb
SKIN CARE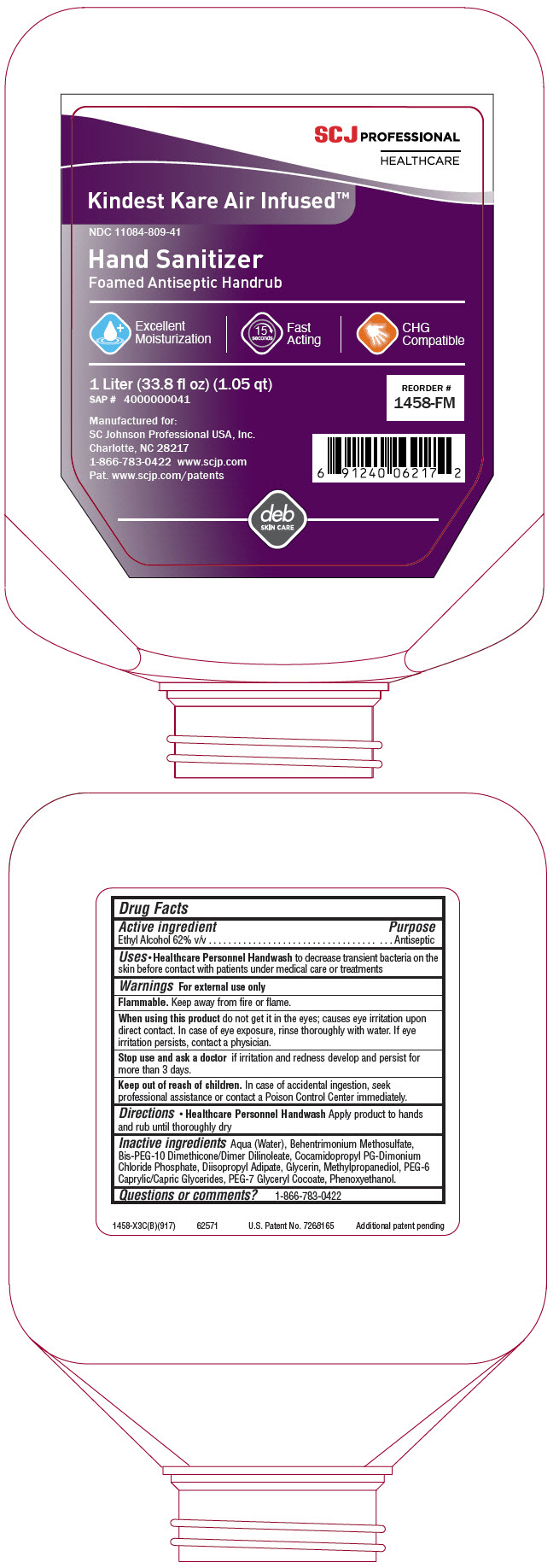
-
INGREDIENTS AND APPEARANCE
KINDEST KARE AIR-INFUSED FOAM ANTISEPTIC HANDRUB
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11084-809 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mg in 100 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Bis-PEG-10 Dimethicone/Dimer Dilinoleate Copolymer (UNII: CF5W1YCX11) methylpropanediol (UNII: N8F53B3R4R) phenoxyethanol (UNII: HIE492ZZ3T) behentrimonium methosulfate (UNII: 5SHP745C61) PEG-7 glyceryl cocoate (UNII: VNX7251543) glycerin (UNII: PDC6A3C0OX) CAPRYLOCAPROYL POLYOXYLGLYCERIDES 6 (UNII: GO50W2HWO8) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11084-809-41 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2018 2 NDC:11084-809-13 444 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2018 04/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 02/01/2018 Labeler - SC Johnson Professional USA, Inc. (607378015) Establishment Name Address ID/FEI Business Operations STERIS Corporation 139424188 MANUFACTURE(11084-809)