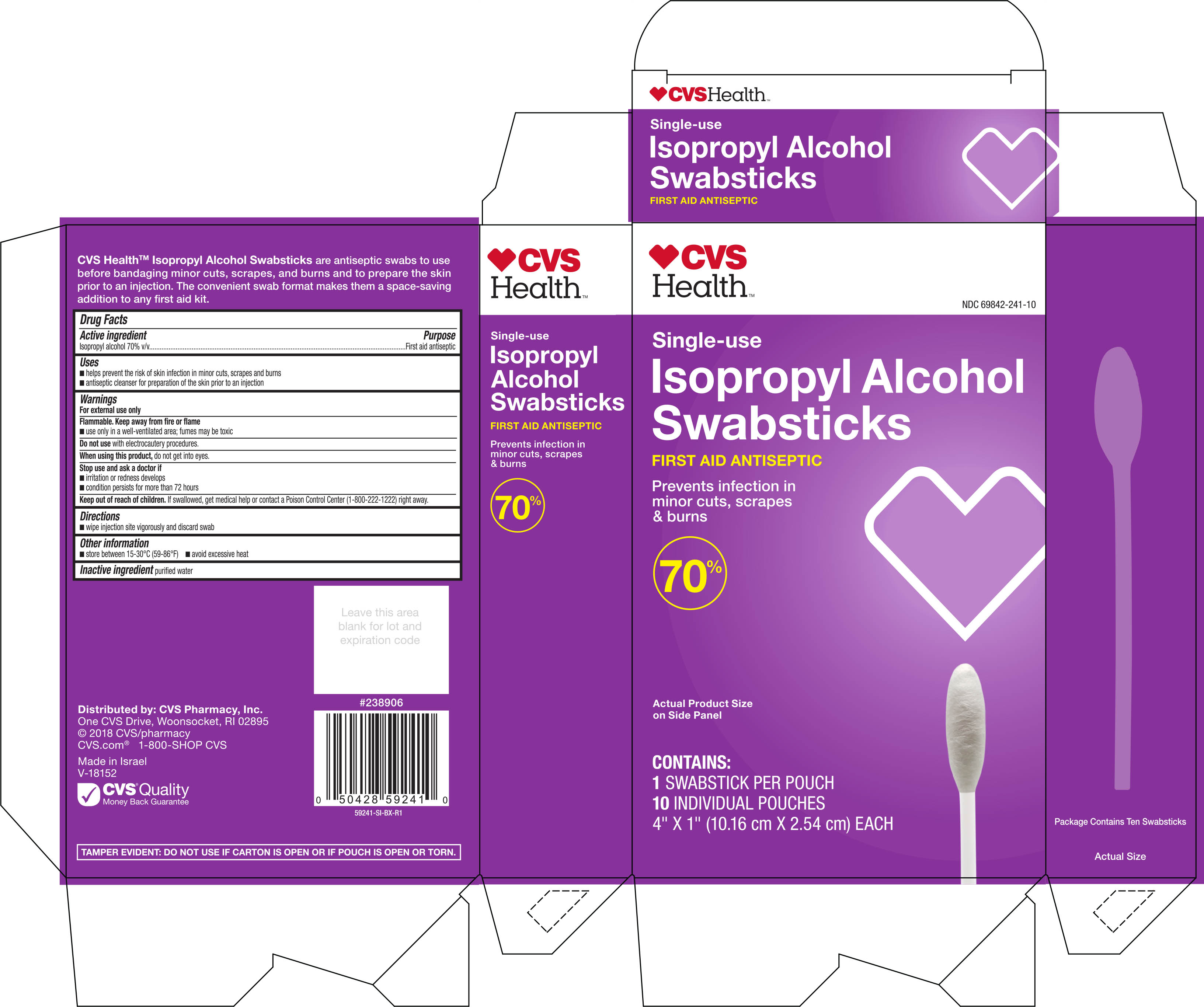
CVS HEALTH ALCOHOL SWABSTICKS- isopropyl alcohol swab
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CVS Health Isopropyl Alcohol Swabsticks Single Use 10ct
Active ingredient Purpose
Isopropyl alcohol 70% v/v...................................................First aid antiseptic
Uses
- helps prevent the risk of skin infection in minor cuts, scrapes, and burns
- antiseptic cleanser for preparation of the skin prior to an injection
Warnings
For external use only
Flammable. Keep away from fire or flame
- use only in a well-ventilated area; fumes may be toxic
Stop use and ask a doctor if
- irritation or redness develops
- condition persists for more than 72 hours
| CVS HEALTH ALCOHOL SWABSTICKS
isopropyl alcohol swab |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
Revised: 12/2021
Document Id: d297b2c7-9f00-e4ab-e053-2a95a90a3145
Set id: 6444c6df-111b-07d5-e053-2991aa0ac219
Version: 4
Effective Time: 20211207
CVS Pharmacy