SODIUM CHLORIDE- sodium chloride injection
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
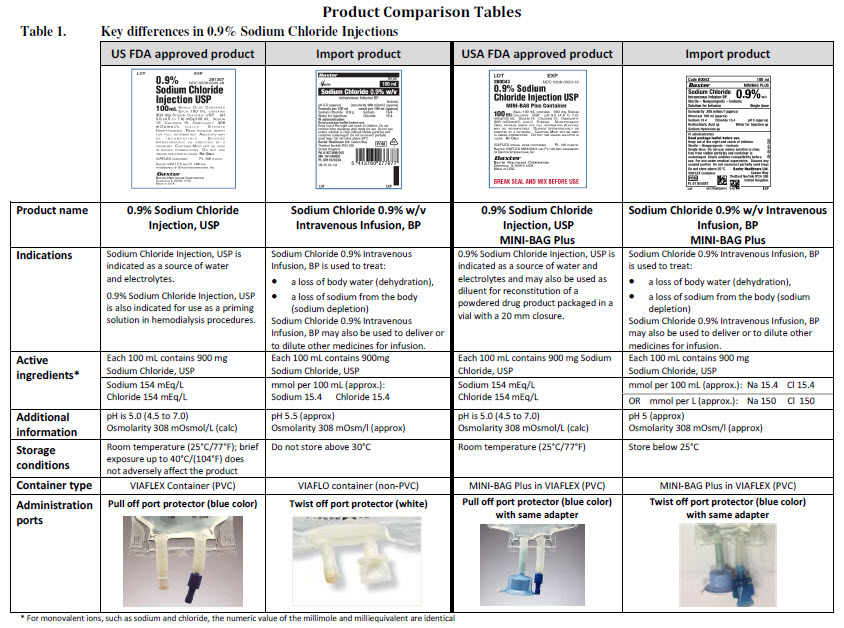
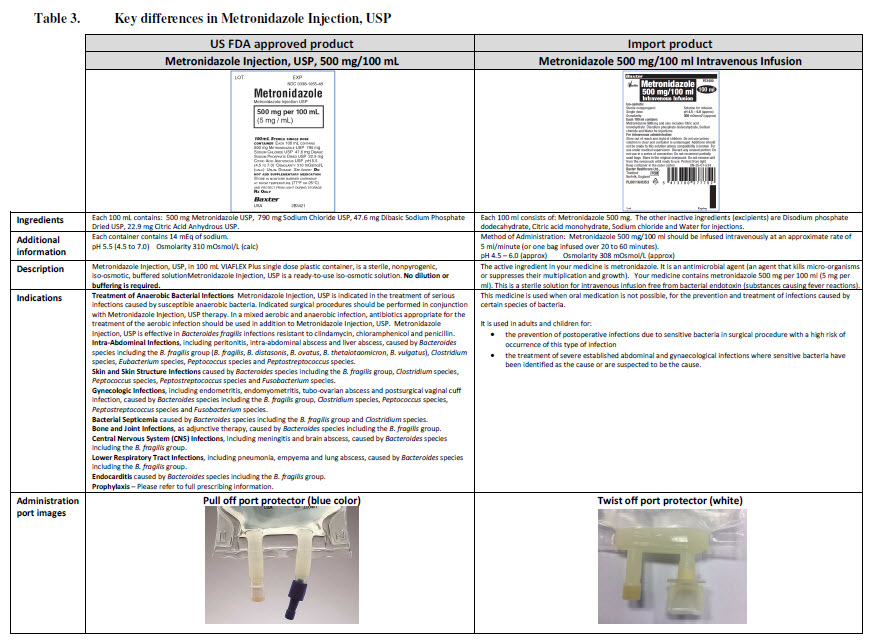
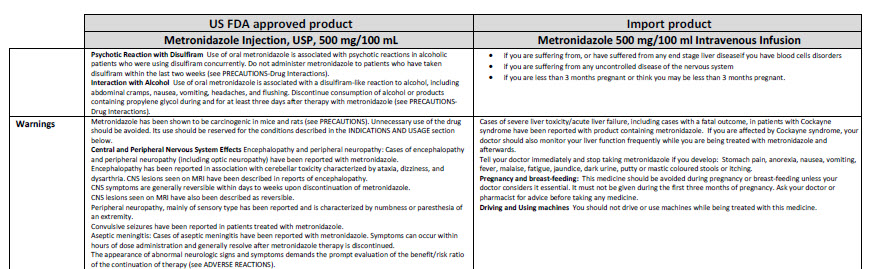
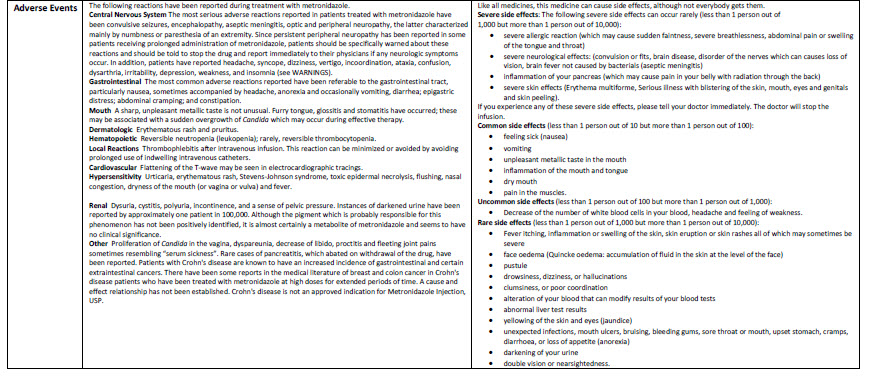
Sodium Chloride 0.9% w/v Intravenous Infusion, BP, in VIAFLO container
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Baxter Logo
FE1306G
Viaflo Logo
50 ml
Sodium Chloride 0.9% w/v
Intravenous Infusion BP
- Isotonic
pH 5.5 (approx)
Osmolarity 308 m0sm/l (approx.)
Formula per 50 ml
mmol/l per 50 ml (approx)
Sodium Chloride 0.45 g
Sodium 7.7
Water for Injections
Chloride 7.7
IV administration
Read package leaflet before use Keep out of sight and
reach of children Do not remove from overwrap until ready
for use Do not use unless solution is clear without visible
particles, and container undamaged Do not reconnect
partially used bags Do not store above 30°C
Baxter Healthcare Ltd Caxton Way
Thetford Norfolk IP24 3SE
United Kingdom
POM symbol
PA167/008/015
MA161/00403
PL00116/0334
UN-35-03-132
0 085412 578453
LOT EXP
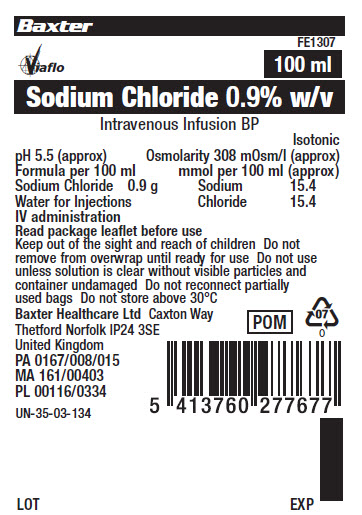
Baxter Logo
FE1307
Viaflo Logo
100 ml
Sodium Chloride 0.9% w/v
Intravenous Infusion BP
- Isotonic
pH 5.5 (approx)
Osmolarity 308 m0sm/l (approx.)
Formula per 100 ml
mmol/l per 100 ml (approx)
Sodium Chloride 0.9 g
Sodium 15.4
Water for Injections
Chloride 15.4
IV administration
Read package leaflet before use
Keep out of sight and reach of children Do not
remove from overwrap until ready for use Do not use
unless solution is clear without visible particles, and
container undamaged Do not reconnect partially
used bags Do not store above 30°C
Baxter Healthcare Ltd Caxton Way
Thetford Norfolk IP24 3SE
United Kingdom
POM symbol
PA167/008/015
MA161/00403
PL00116/0334
UN-35-03-134
5413760277677
LOT EXP
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare S.A. | 988899845 | ANALYSIS(0338-9546, 0338-9550) , LABEL(0338-9546, 0338-9550) , MANUFACTURE(0338-9546, 0338-9550) , PACK(0338-9546, 0338-9550) , STERILIZE(0338-9546, 0338-9550) | |