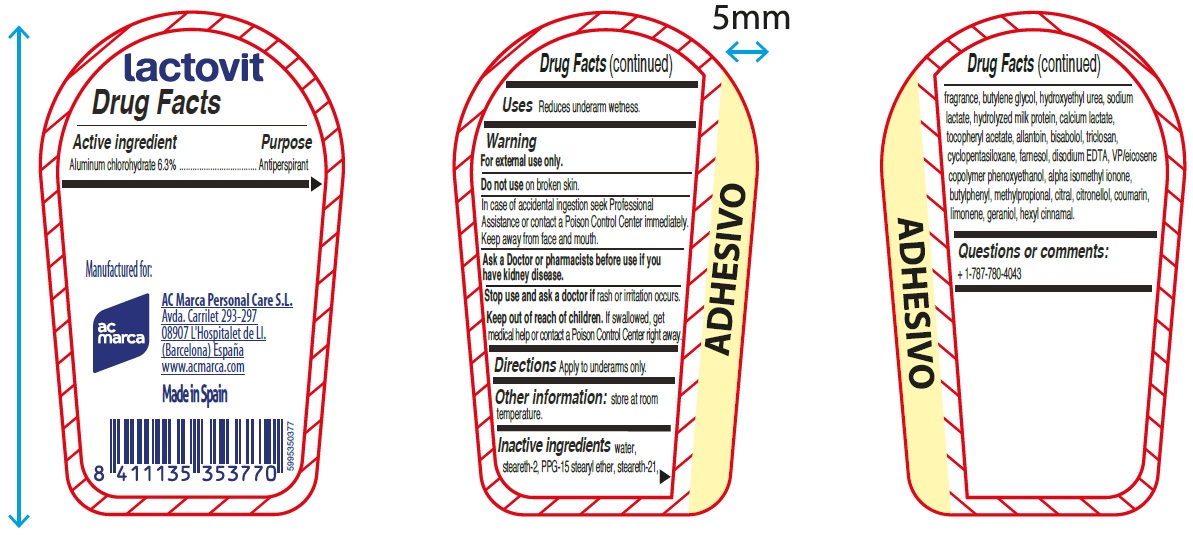
LACTOVIT LACTOUREA DEODORANT AND ANTIPERSPIRANT- aluminum chlorohydrate cream
AC Marca Personal Care
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
LACTOVIT LACTOUREA DEODORANT AND ANTIPERSPIRANT
Warning
For external use only.
Inactive ingredients
water, steareth-2, PPG-15 stearyl ether, steareth-21, fragrance, butylene glycol, hydroxyethyl urea, sodium lactate, hydrolyzed milk protein, calcium lactate, tocopheryl acetate, allantoin, bisabolol, triclosan, cyclopentasiloxane, farnesol, disodium EDTA, VP/eicosene copolymer phenoxyethanol, alpha isomethyl ionone,butylphenyl, methylpropional, citral, citronellol, coumarin, limonene, geraniol, hexyl cinnamal.
| LACTOVIT LACTOUREA DEODORANT AND ANTIPERSPIRANT
aluminum chlorohydrate cream |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - AC Marca Personal Care (460005267) |