Label: ACETAMIOPHEN- acetaminophen tablet, extended release
- NDC Code(s): 23155-202-01, 23155-202-18, 23155-202-64, 23155-202-65
- Packager: Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
- PURPOSE
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product.
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash.
If a skin reaction occurs, stop use and seek medical help right away.
- Do not use
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- do not take more than directed (see overdose warning)
Adults and children 12 years and over:
- take 2 caplets every 8 hours with water
- swallow whole; do not crush, chew, split or dissolve
- do not take more than 6 caplets in 24 hours
- do not use for more than 10 days unless directed by a doctor
- do not use
- Other information
- Inactive ingredients
- Questions or comments?
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL Carton
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 24 Caplets Label
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 100 Caplets label
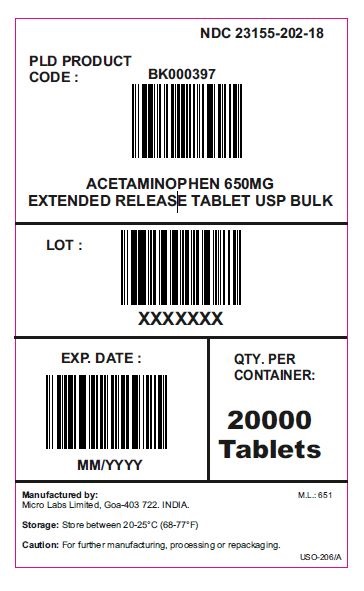
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 20000 Tablets
-
INGREDIENTS AND APPEARANCE
ACETAMIOPHEN
acetaminophen tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:23155-202 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE (140 CPS AT 5%) (UNII: 8136Y38GY5) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color WHITE (caplet) Score no score Shape CAPSULE (yellow layer and white coloured plain layer) Size 19mm Flavor Imprint Code 511 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23155-202-64 1 in 1 CARTON 06/01/2018 1 24 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:23155-202-65 1 in 1 CARTON 06/01/2018 2 750 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:23155-202-01 1 in 1 CARTON 06/01/2018 3 100 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:23155-202-18 20000 in 1 CONTAINER; Type 0: Not a Combination Product 06/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207035 06/01/2018 Labeler - Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc. (780779901) Registrant - Heritage Pharma Labs Inc. d/b/a Avet Pharmaceuticals Labs Inc. (189630168) Establishment Name Address ID/FEI Business Operations Micro Labs Limited 915793658 MANUFACTURE(23155-202)