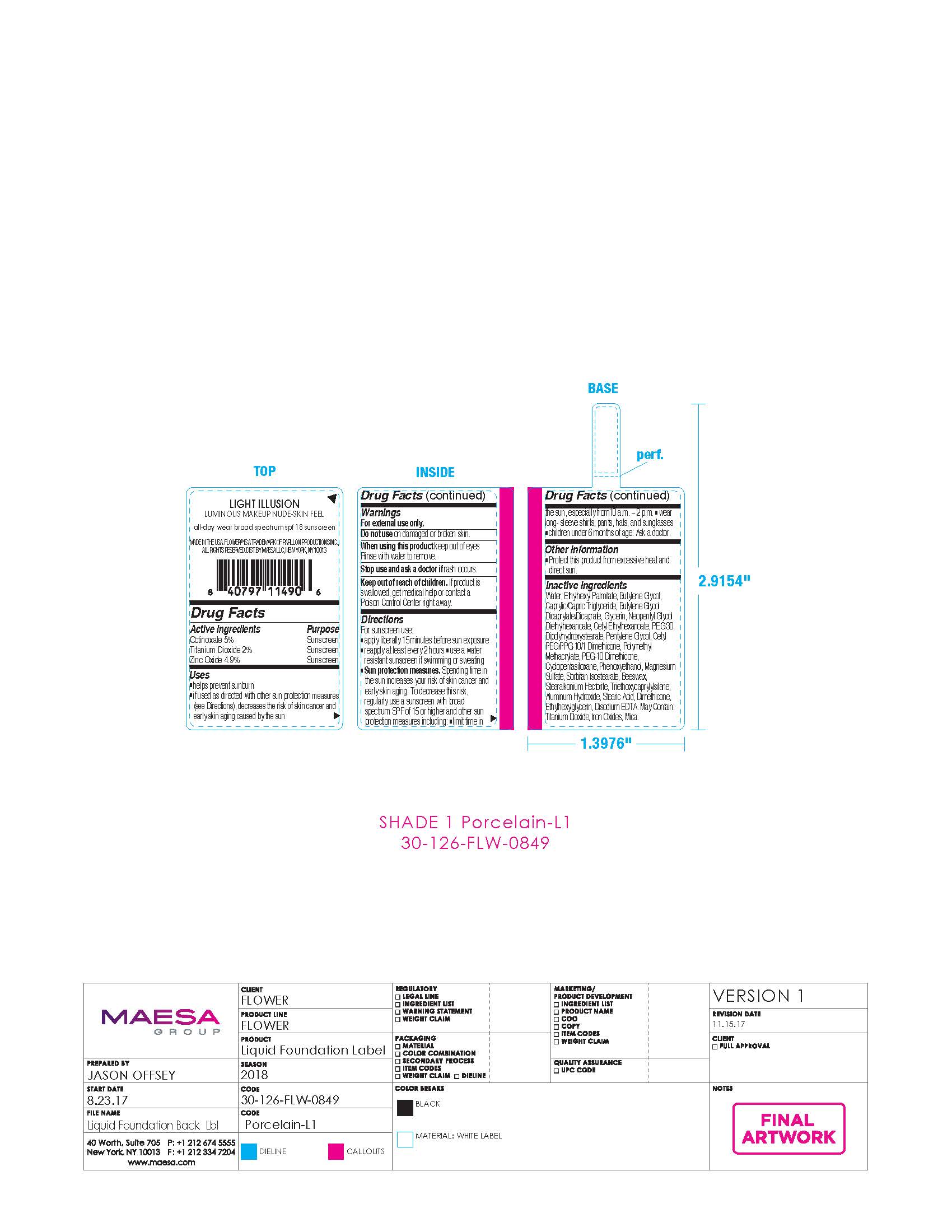
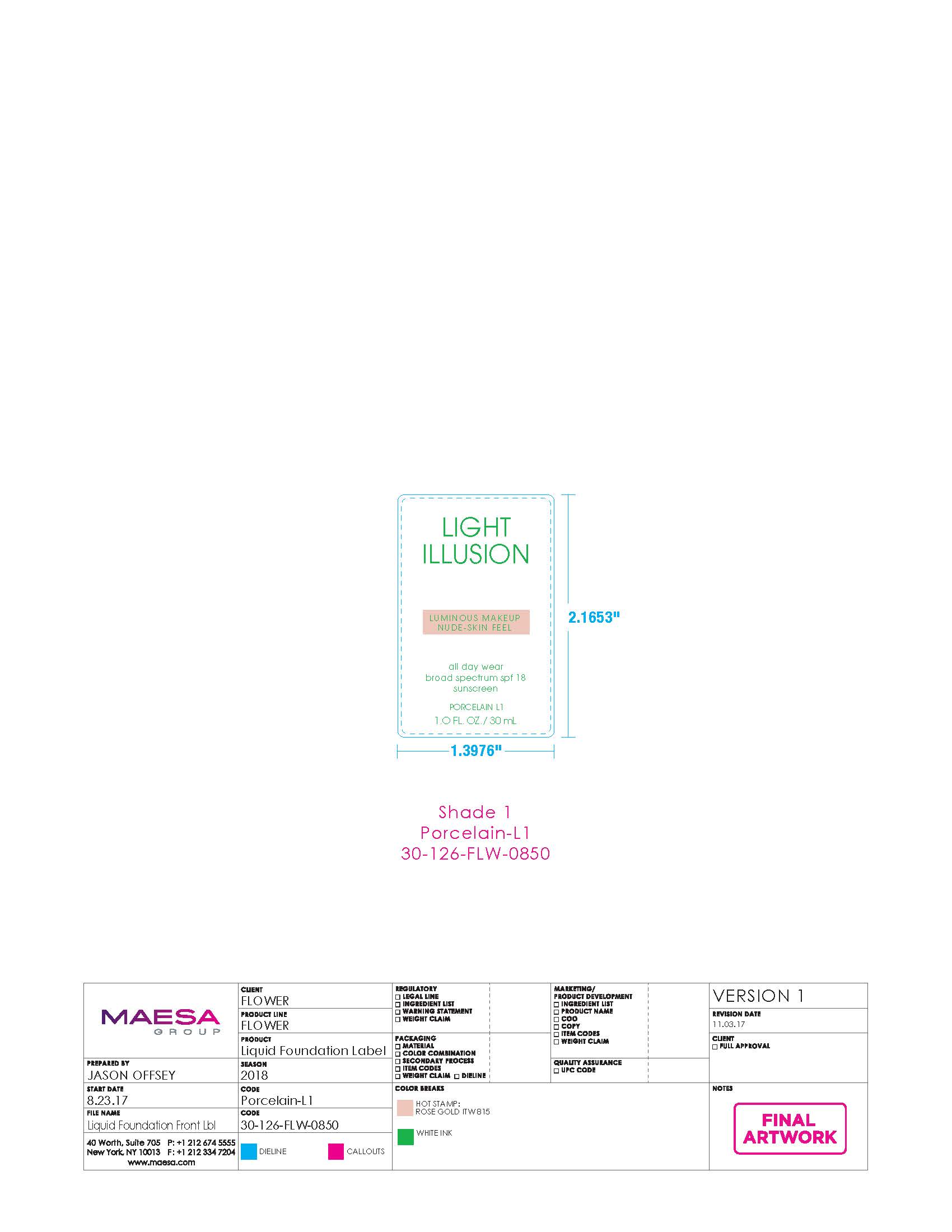
LIGHT ILLUSION LUMINOUS MAKEUP NUDE-SKIN FEEL ALL-DAY WEAR BROAD SPECTRUM SPF18 SUNSCREEN 01- octinoxate, titanium dioxide, zinc oxide cream
MAESA LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| LIGHT ILLUSION LUMINOUS MAKEUP NUDE-SKIN FEEL ALL-DAY WEAR BROAD SPECTRUM SPF18 SUNSCREEN 01
octinoxate, titanium dioxide, zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - MAESA LLC (144282311) |
| Registrant - COSMAX USA, INC. (COSMAX USA, CORPORATION) (010990210) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| COSMAX USA, INC. (COSMAX USA, CORPORATION) | 010990210 | manufacture(71899-001) | |
Revised: 7/2023
Document Id: 01b6a8fc-6927-5680-e063-6294a90ad74d
Set id: 638e119f-51c8-59a6-e053-2991aa0aeffa
Version: 2
Effective Time: 20230730
MAESA LLC