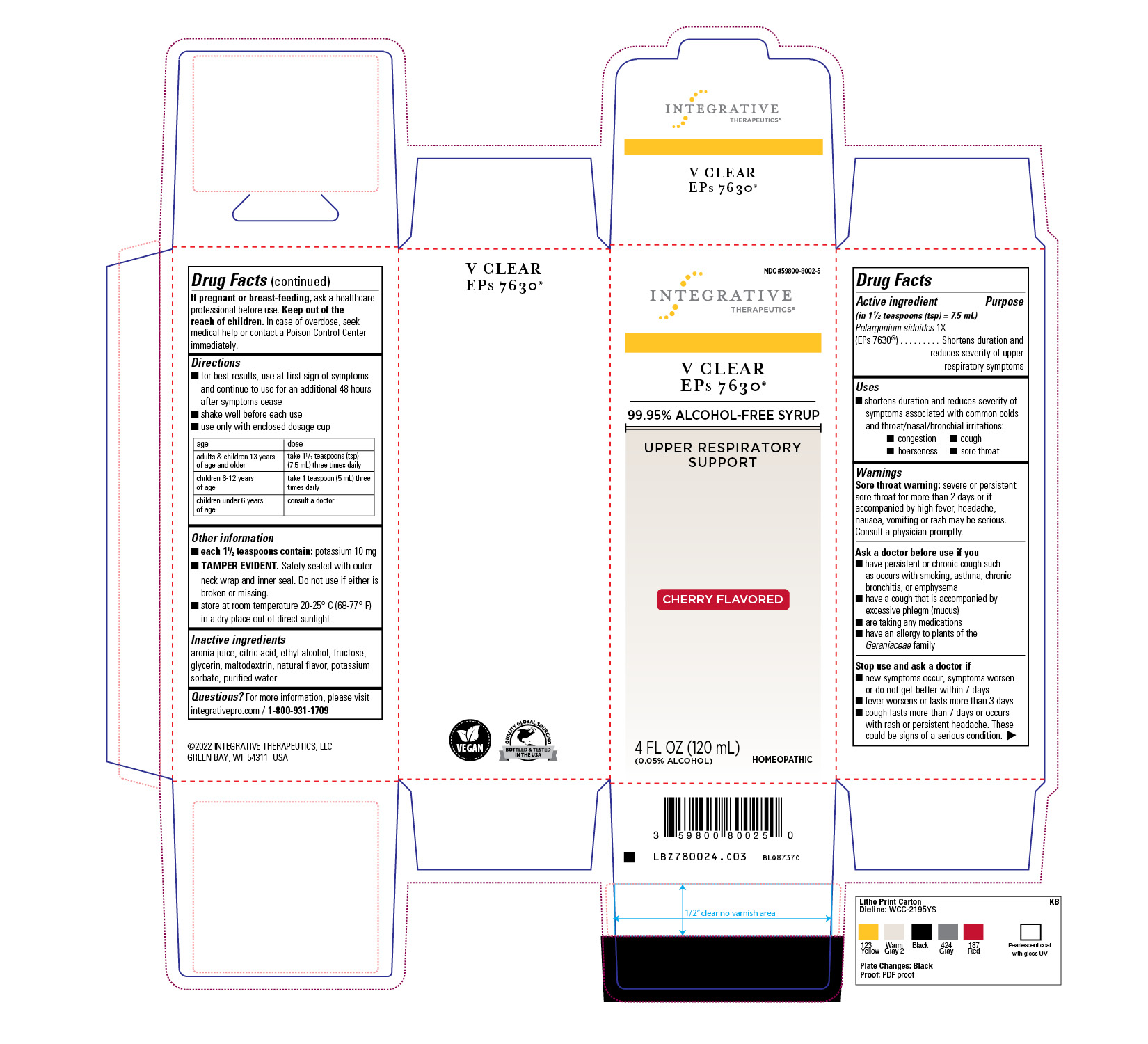
Label: VIRACLEAR EPS 7630 CHERRY FLAVOR- pelargonium sidoides liquid
- NDC Code(s): 59800-8002-5
- Packager: Schwabe North America, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
-
Dosage & Administration
For best results, use at first sign of symptoms and continue to use for an additional 48 hours after symptoms cease.
Shake well before each use.
Use only with enclosed dosage cup.
adults & children 13 years of age and older: take 1-1/2 teaspoons (tsp) (7.5 mL) three times daily
children 6-12 years of age: take 1 teaspoon (5 mL) three times daily
children under 6 years of age: consult a doctor
- Indications and Usage
- Purpose
-
Warning
Sore throat warning: severe or persistent sore throat for more than 2 days or if accompanied by high fever, headache, nausea, vomiting or rash may be serious. Consult a physician promptly.
Ask the Doctor
Ask a doctor before use if you have a persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
have a cough that is accompanied by excessive phlegm (mucus)
are taking any medications
have an allergy to plants of the Geraniaceae family
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VIRACLEAR EPS 7630 CHERRY FLAVOR
pelargonium sidoides liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59800-8002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PELARGONIUM SIDOIDES ROOT (UNII: H6J53HEX8E) (PELARGONIUM SIDOIDES ROOT - UNII:H6J53HEX8E) PELARGONIUM SIDOIDES ROOT 1 [hp_X] in 120 mL Inactive Ingredients Ingredient Name Strength ARONIA MELANOCARPA FRUIT JUICE (UNII: D2EVP827PJ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALCOHOL (UNII: 3K9958V90M) FRUCTOSE (UNII: 6YSS42VSEV) MALTODEXTRIN (UNII: 7CVR7L4A2D) WATER (UNII: 059QF0KO0R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color Score Shape Size Flavor CHERRY (NATURAL CHERRY FLAVOR) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59800-8002-5 1 in 1 CARTON 08/24/2009 1 120 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/24/2009 Labeler - Schwabe North America, Inc (831153908) Establishment Name Address ID/FEI Business Operations Schwabe North America, Inc 831153908 manufacture(59800-8002)