DAYTIME COLD AND FLU RELIEF- acetaminophen, dextromethorphan hbr, phenylephrine hci capsule, liquid filled
AiPing Pharmaceutical, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Daytime Cold and Flu Relief
ACTIVE INGREDIENTS (IN EACH SOFTGEL)
Acetaminophen 325mg
Dextromethorphan HBr 10mg
Phenylephrine HCI 5mg
USES
temporarily relieves common cold/flu symptoms:
- nasal congestion
- cough due to minor throat & bronchial irritation
- sore throat
- headache
- minor aches & pains
- fever
WARNINGS
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4 doses in 24 hrs, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks daily while using this product
Sore throat warning
If sore throat is severe, lasts for more than 2 days, occurs with or is followed by fever, headache, rash, nausea, or vomiting, see a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- thyroid disease
- diabetes
- high blood pressure
- trouble urinating due to enlarged prostate gland
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough as occurs with smoking, asthma, or emphysema
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- symptoms get worse or last more than 5 days (children) or 7 days (adults)
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back, or occurs with rash or headache that lasts. These could be signs of a serious condition.
DIRECTIONS
- take only as directed - see Overdose warning
- do not exceed 4 doses per 24 hrs
| adults & children 12 yrs & over | 2 softgels with water every 4 hrs |
| children 4 to under 12 yrs | ask a doctor |
| children under 4 yrs |
do not use |
- when using other Daytime or Nighttime products, carefully read each label to insure correct dosing
OTHER INFORMATION
- store at room temperature
TAMPER EVIDENT: This package is safety sealed & child resistant. Use only if blisters are intact. If difficult to open, use scissors.
INACTIVE INGREDIENTS
FD&C red #40, FD&C yellow #6, gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, sorbitol sorbitan solution and white edible ink
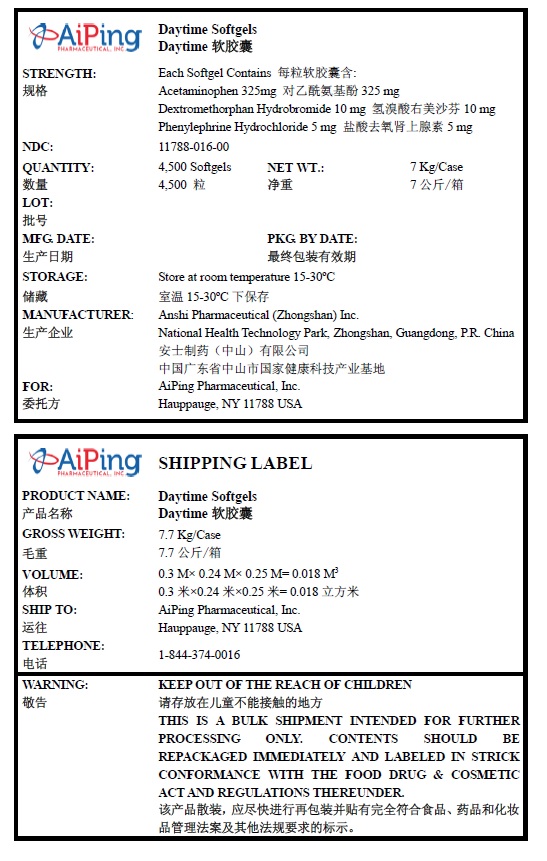
PRINCIPAL DISPLAY PANEL
Daytime Softgels
NDC: 11788-016-00
Quantity: 4,500 Softgels
WARNING:
KEEP OUT OF THE REACH OF CHILDREN. THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY. CONTENTS SHOULD BE REPACKAGED IMMEDIATELY AND LABELED IN STRICK CONFORMANCE WITH THE FOOD DRUG & COSMETIC ACT AND REGULATIONS THEREUNDER.
| DAYTIME COLD AND FLU RELIEF
acetaminophen, dextromethorphan hbr, phenylephrine hci capsule, liquid filled |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - AiPing Pharmaceutical, Inc. (079674526) |
| Registrant - AiPing Pharmaceutical, Inc. (079674526) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Anshi Pharmaceutical (Zhongshan) Inc. | 528101821 | manufacture(11788-016) | |