ACLARO HYDROQUINONE- hydroquinone emulsion
Innocutis
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Aclaro Emulsion
Description
Rx only
For topical use only
Not for ophthalmic use
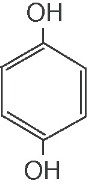
Hydroquinone is 1,4-benzenediol. Hydroquinone is structurally related to monobenzone. Hydroquinone occurs as fine, white needles. The drug is freely soluble in water and in alcohol with a pKa of 9.96. Chemically, h ydroquinone is designated as p-dihydroxybenzene; the empirical formula is C6 H6 O2; molecular weight 110.1.The structural formula is:
Active Ingredients: hydroquinone USP 4% Other Ingredients: ascorbic acid, benzyl alcohol, butyl methoxydibenzoylmethane, C12-15 alkyl benzoate, cetearyl octanoate, cetyl alcohol, cetyl esters, cetyl palmitate, DEA cetyl phosphate, dimethicone, disodium EDTA, ethylhexyl methoxycinnamate, glycerin, glycolic acid, ammonium glycolate, hydroxyethylcellulose, phenoxyethanol, purified water, sodium metabisulfite, and stearic
acid.
Clinical Pharmacology
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3- (3,4-dihydroxyphenyl) alanine (dopa) and suppression of other melanocyte metabolic processes. 2
Indications and Usage
Aclaro® is indicated for the gradual treatment of ultraviolet induced dyschromia and discoloration resulting rom the use of oral contraceptives, pregnancy, hormone replacment therapy, or skin trauma.
Contraindications
Aclaro® is contraindicated in any patient that has a prior history of hypersensitivity or allergic reaction to hydroquinone or any of the other ingredients. The safety of topical hydroquinone use during pregnancy or on children (12 years and under) has not been established.
Warnings
A. Caution: Hydroquinone is a depigmenting agent which may produce unwanted cosmetic effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this medication.
B.Test for skin sensitivity before using Aclaro® (hydroquinone USP 4%) emulsion by applying a small amount to an unbroken patch of skin and check within 24 hours. Minor redness is not a contraindication, but where there is itching, vesicle formation, or excessive inflammatory response, further treatment is not advised. Close patient supervision is recommended. Contact with the eyes should be avoided. If no lightening effect is noted after two months of treatment, use of Aclaro® emulsion should be discontinued.
C. Sunscreen use is an essential aspect of hydroquinone therapy because even minimal sunlight exposure sustains melanocyte activity. The sunscreens in Aclaro® emulsion provide the necessary sun protection during therapy. During and after the use of Aclaro® emulsion, sun exposure should be limited or sun-protective clothing should be used to cover the treated areas to prevent repigmentation.
D. Keep this and all medications out of the reach of children. In case of accidental ingestion, contact a physician or poison control center immediately.
E. Contains sodium metabisulfite, a sulfite that may cause serious allergic reactions (e.g., hives, itching, wheezing, anaphylaxis, severe asthma attack) in certain susceptible persons.
F. On rare occasions, a gradual blue-black darkening of the skin may occur. If this occurs, the product should be discontinued and a physician contacted immediately.
Pregnancy Category C
Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether hydroquinone can cause fetal harm when used topically on a pregnant woman or affect reproductive capacity. It is not known to what degree, if any, topical hydroquinone is absorbed systemically. Topical hydroquinone should be used in pregnant women only when clearly indicated.
Nursing Mothers
Nursing mothers: It is not known whether topical hydroquinone is absorbed or excreted in human milk. Caution is advised when hydroquinone is used by a nursing mother.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Pediatric usage
Safety and effectiveness in pediatric patients below the age of 12 years have not been established.
Dosage and Administration
Apply Umecta topical suspension to affected skin twice per day or as directed by a physician. Rub in until completely absorbed.
Adverse Reactions
No systemic reactions have been reported. Occasional cutaneous hypersensitivity (localized contact dermatitis) may occur, in which case the medication should be discontinued and the physician notified immediately.
Overdosage Section
There have been no systemic reactions reported from the use of topicalshydroquinone.However, treatment should be limited to relatively small areas of thesbody at one time, since some patients experience a transient skin reddening and a mildsburning sensation which does not preclude treatment.
Dosage and Administration
Aclaro® emulsion should be applied to the affected areas twice daily, or as directed by a physician. There is no recommended dosage for pediatric patients under 12 years of age except under the advice and supervision of a physician.
How Supplied
Aclaro®(hydroquinone USP 4%) emulsion is available as follows:
1.7 ounce airless pump bottle NDC 68712-003-01
Store at controlled room temperature:15˚ - 25˚ C (59˚ – 77˚ F)
Manufactured for:
Innocutis Holdings, LLC.
Charleston, SC 29401
www.innocutis.com
www.Aclaro4.com
1. Denton C., Lerner A.B., and Fitzpatrick T.B. "Inhibition of Melanin Formation by Chemical Agents." Journal of Investigative Dermatology. 1952;18:119 - 135.
2. Jimbow K., Obata H., Pathak M., and Fitzpatrick T.B. "Mechanism of Depigmentation by Hydroquinone." Journal of Investigative Dermatology. 1974;62:436 - 449.
| ACLARO
HYDROQUINONE
hydroquinone emulsion |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Innocutis (071501252) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ei Inc. | 105803274 | manufacture(68712-003) | |
