PCA SKIN ACNE- salicylic acid gel
CP Skin Health group, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
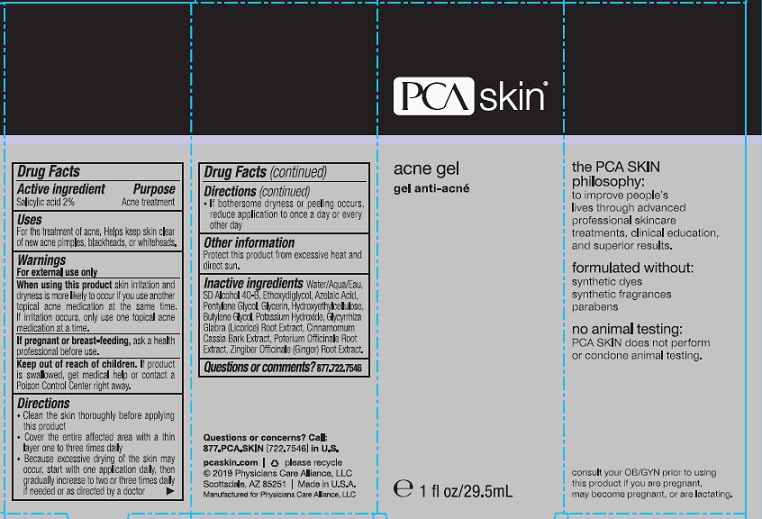
PCA SKIN Acne Gel
Uses:
- Clears and reduces the severity of acne blemished
- penetrates pores to help prevent development of new blemishes/blackheads.
Warnings: For external use only
_____________________________
Using other topical acne drugs at the same time or immediately following the use of this product may increase skin dryness/ irritation. If this occurs, use only one acne drug unless directed by a physician.
_______________________________
Do not use in or near the eyes
Directions:
- Cleanse skin before applying
- Apply a thin layer to affected areas.
- Do not rinse
- Apply once daily. Gradually increase to twice daily or as directed by a physician. If excessive dryness or redness occurs, reduce application.
Inactive Ingredients:
Water/Aqua/Eau, SD Alcohol 40-B, Ethoxydiglycol, Azelaic Acid, Pentylene Glycol, Glycerin, Hydroxyethylcellulose, Butylene Glycol, Potassium Hydroxide, Glycyrrhiza Glabra (Licorice) root extract, Poterium Officinale (Green Burnet) Root, Cinnamomum Cassia Bark, Zingiber Officinale (Ginger) Root.
A gentle and effective 2% salicylic acid acne spot treatment that clears existing acne blemishes while reducing oil production and the occurence of future breakouts
Un traitement doux et effectif a base de 2 % d'acide salicylique pour les boutons d'acne, reduit la production de sebum et la fromation de futures lesions.
pcaskin.com please recycle
2008 Physicians Care Alliance, LLC
Scottsdale, AZ 85253 Made in U.S.A
#21175
| PCA SKIN ACNE
salicylic acid gel |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - CP Skin Health group, Inc (611921669) |