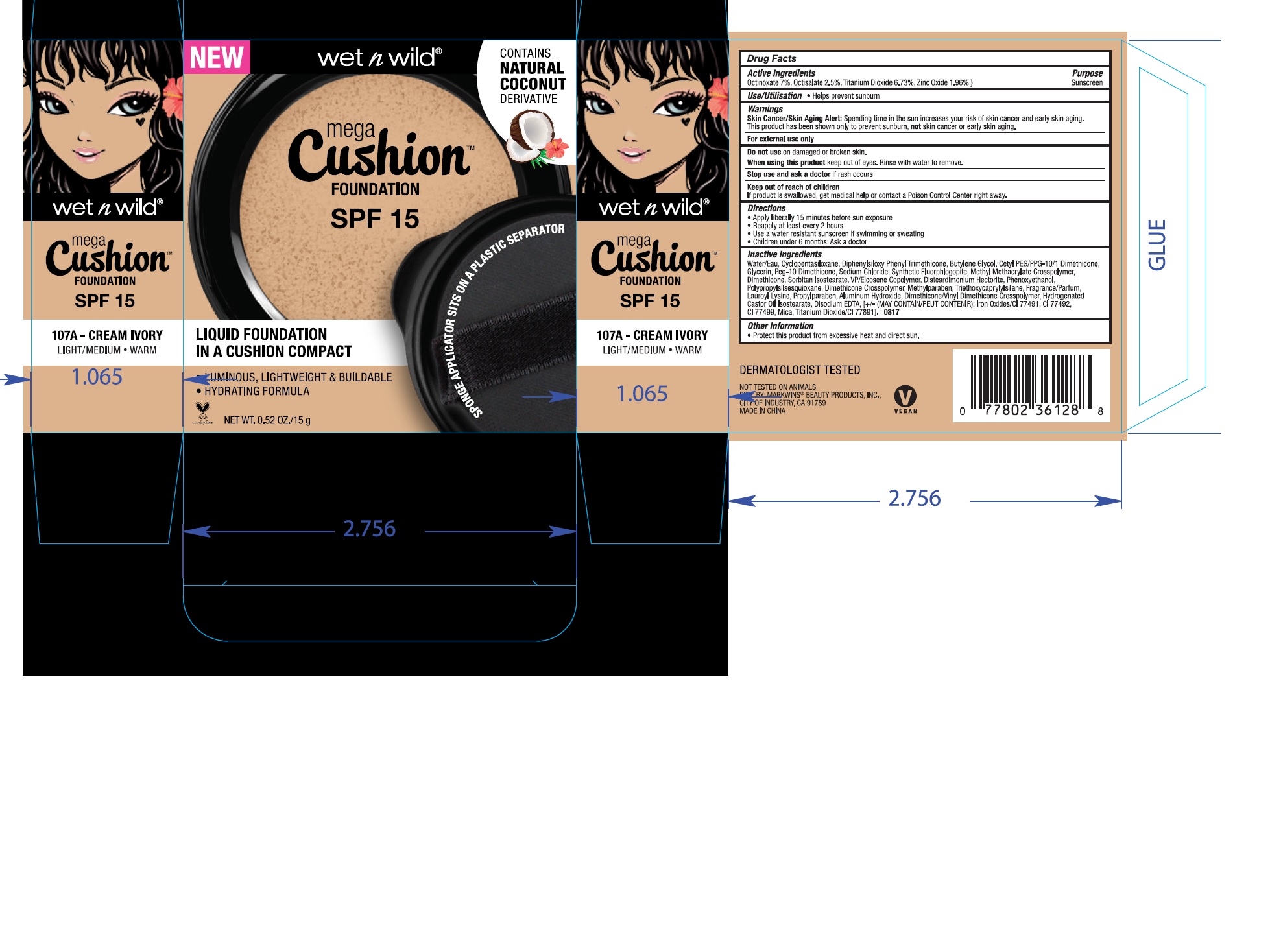
MEGACUSHION FOUNDATION SPF 15- octinoxate, octisalate, titanium dioxide, zinc oxide liquid
Markwins Beauty Products, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Megacushion Foundation
| MEGACUSHION FOUNDATION
SPF 15
octinoxate, octisalate, titanium dioxide, zinc oxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Markwins Beauty Products, Inc. (135407760) |
Revised: 4/2020
Document Id: a382e522-56da-fae5-e053-2995a90a84d0
Set id: 6289bfa9-1730-e564-e053-2a91aa0aa0b7
Version: 3
Effective Time: 20200417
Markwins Beauty Products, Inc.