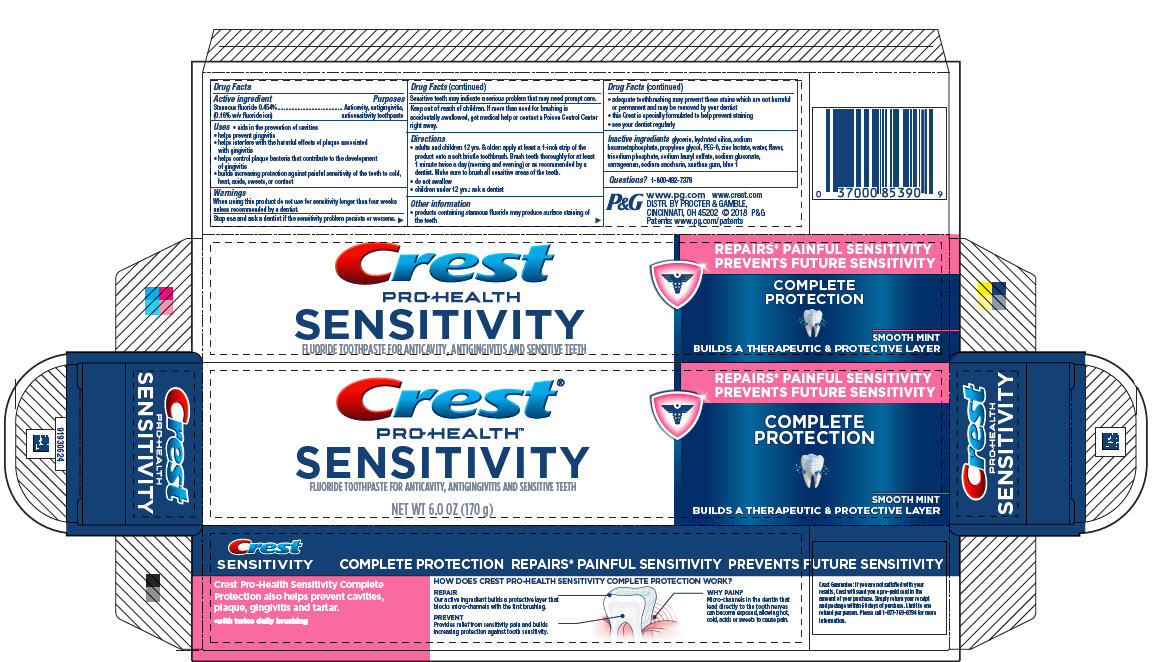
Label: CREST SENSITVITY COMPLETE PROTECTION- stannous fluoride paste, dentifrice
- NDC Code(s): 37000-899-06, 37000-899-35
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purposes
-
Uses
- aids in the prevention of cavities
- helps prevent gingivitis
- helps interfere with the harmful effects of plaque associated with gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets, or contact
- Warnings
-
Directions
- adults and children 12 yrs. & older: apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist. Make sure to brush all sensitive areas of the teeth.
- do not swallow
- children under 12 yrs.: ask a dentist
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 170g Tube Carton
-
INGREDIENTS AND APPEARANCE
CREST SENSITVITY COMPLETE PROTECTION
stannous fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37000-899 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.6 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) WATER (UNII: 059QF0KO0R) ZINC LACTATE (UNII: 2GXR25858Y) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM GLUCONATE (UNII: R6Q3791S76) CARRAGEENAN (UNII: 5C69YCD2YJ) SACCHARIN SODIUM (UNII: SB8ZUX40TY) XANTHAN GUM (UNII: TTV12P4NEE) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) SODIUM PHOSPHATE, TRIBASIC, ANHYDROUS (UNII: SX01TZO3QZ) Product Characteristics Color green Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37000-899-35 1 in 1 CARTON 01/16/2018 09/02/2020 1 99 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:37000-899-06 1 in 1 CARTON 01/16/2018 2 170 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 01/16/2018 Labeler - The Procter & Gamble Manufacturing Company (004238200)