MOUTH FRESH- cetylpyridinium chloride spray
Yuyao Jessie Commodity Co.,Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
mouth fresh 500-501
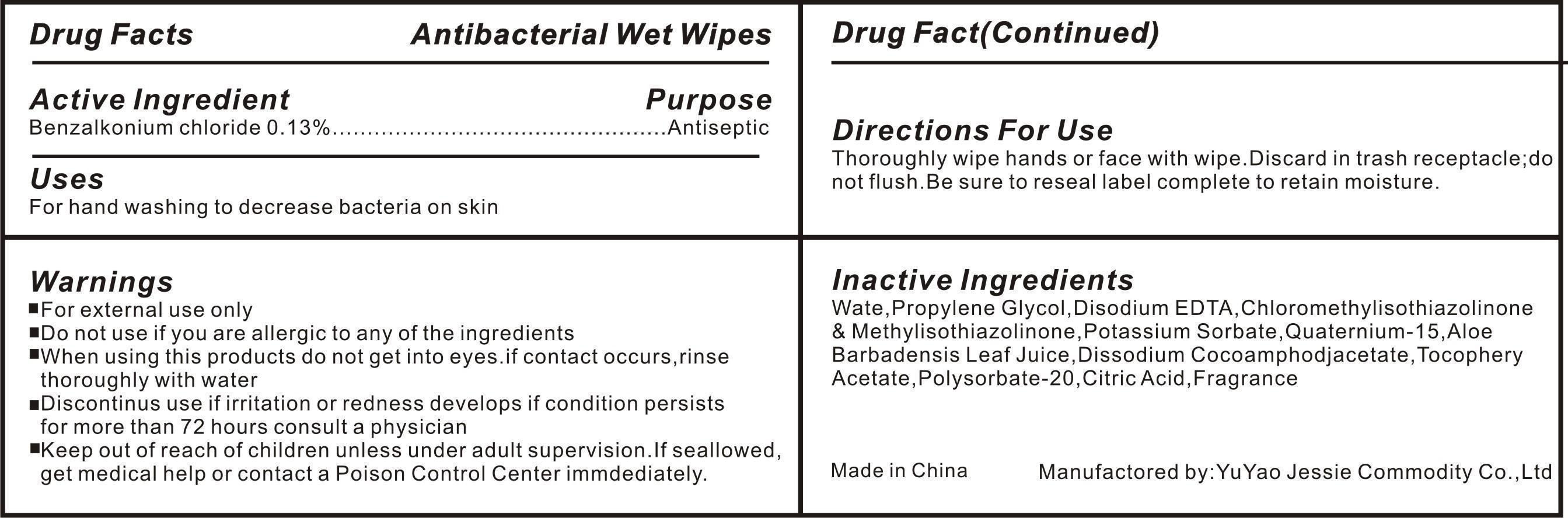
WARNINGS
For External use only.
Do not use if you are allergic to any of the ingredients.
When using this product Do not get into eyes,if contact occurs ,rinse throughly with water.
Discontinue use if irritation or redness develops if condition persists for more than 72 hours consults a physician.
Directions
Open Cap to on position exposing nozzle Pump 1-2 sprays directly into mouth.
Children under 6 months of age:ask a doctor
| MOUTH FRESH
cetylpyridinium chloride spray |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Yuyao Jessie Commodity Co.,Ltd. (529892305) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Yuyao Jessie Commodity Co.,Ltd. | 529892305 | manufacture(51414-501) | |
Revised: 4/2019
Document Id: 873286a0-b064-4d8a-e053-2a91aa0a5876
Set id: 6259609f-8625-1472-e053-2991aa0a10a2
Version: 2
Effective Time: 20190423
Yuyao Jessie Commodity Co.,Ltd.