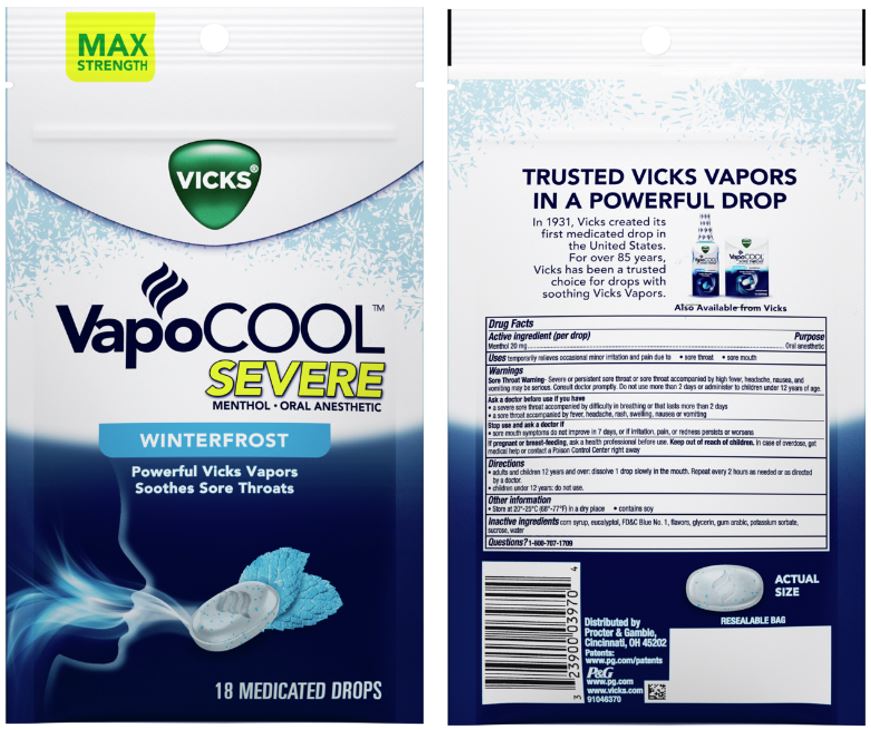
VICKS VAPOCOOL SEVERE- menthol lozenge
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Vicks
® VapoCOOL
™
Severe
Warnings
Sore Throat Warning
Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult doctor promptly. Do not use more than 2 days or administer to children under 5 years of age unless directed by doctor.
Ask a doctor before use if you have
- a severe sore throat accompanied by difficulty in breathing or that lasts more than 2 days
- a sore throat accompanied by fever, headache, rash, swelling, nausea or vomiting
Directions
- adults and children 12 years and over: dissolve 1 drop slowly in the mouth. Repeat every 2 hours as needed or as directed by a doctor.
- children 12 years and under: do not use
Other information
- Store at 20°-25°C (68°-77°F) in a dry place
- Contains Soy. May contain Peanuts, Milk,Wheat, Nuts or Tree Nuts
| VICKS VAPOCOOL
SEVERE
menthol lozenge |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
Revised: 8/2023
Document Id: 039a2036-54b1-cf28-e063-6294a90a8ce6
Set id: 6249abb0-8972-16a5-e053-2991aa0a09fb
Version: 7
Effective Time: 20230823
The Procter & Gamble Manufacturing Company