Label: DE LA CRUZ CAMPHOR- camphor ointment
- NDC Code(s): 24286-1521-2, 24286-1521-5
- Packager: DLC Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
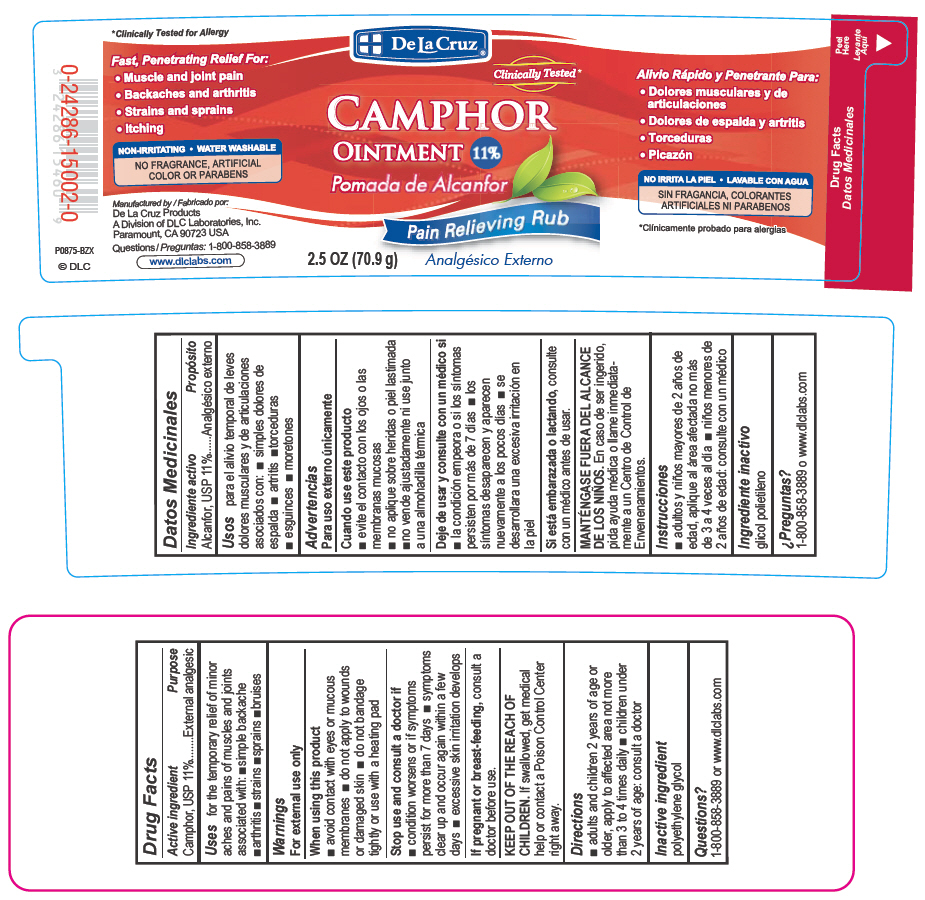
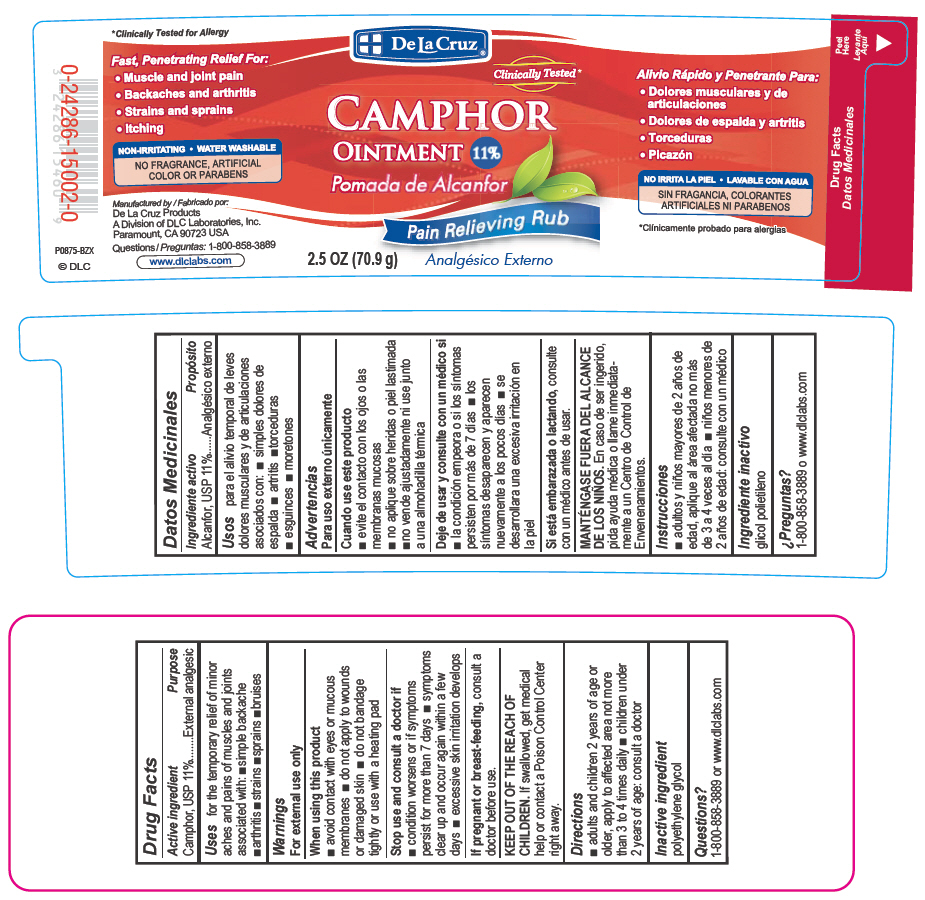
- Active Ingredient
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and consult a doctor if
- KEEP OUT OF THE REACH OF CHILDREN.
- Directions
- Inactive ingredient
- Questions?
-
SPL UNCLASSIFIED SECTION
De La Cruz
CAMPHOR
Ointment 11%Pain relieving rub
2.5 OZ (70.9g)
FAST, PENETRATING RELIEF FOR:
Muscle and joint pain
Backaches and arthritis
Strains and sprains
Itching
NON-IRRITATING
WATER WASHABLE
NO PARABENS OR ARTIFICIAL FRAGRANCES OR COLORS
Manufactured by:
De La Cruz Products
A Division of DLC Laboratories, Inc.
Paramount, CA 90723 USA
Questions: 1-800-858-3889
www.dlclabs.com (c) DLC
- PRINCIPAL DISPLAY PANEL - 70.9 g Jar Label
-
INGREDIENTS AND APPEARANCE
DE LA CRUZ CAMPHOR
camphor ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24286-1521 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 11 g in 100 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24286-1521-2 70.9 g in 1 JAR; Type 0: Not a Combination Product 07/26/2012 2 NDC:24286-1521-5 155.9 g in 1 JAR; Type 0: Not a Combination Product 03/10/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 07/26/2012 Labeler - DLC Laboratories, Inc. (093351930) Establishment Name Address ID/FEI Business Operations DLC Laboratories, Inc. 093351930 manufacture(24286-1521) , label(24286-1521)