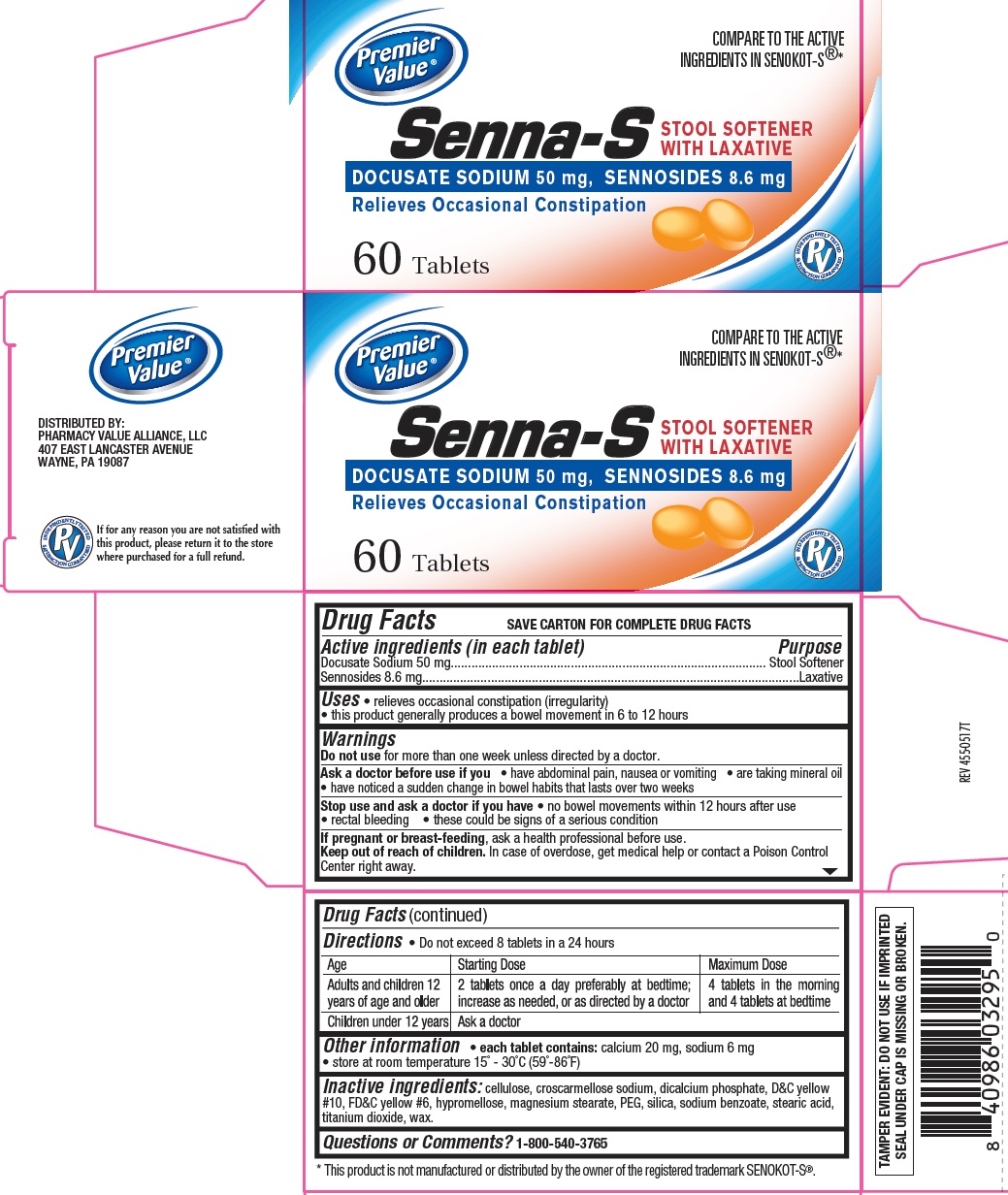
SENNA-S- sennosides and docusate sodium tablet
Chain Drug Consortium, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
pv 455
Uses
- relieves occasional constipation (irregularity)
- this product generally produces a bowel movement in 6 to 12 hours
Warnings
Do not use for more than 1 week unless directed by a doctor
Ask a doctor before use if you
- have abdominal pain, nausea or vomiting
- are taking mineral oil
- have noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if you have
- no bowel movement within 12 hours
- rectal bleeding
- these could signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Directions
• do not exceed 8 tablets in 24 hours
| Age
| Starting Dose
| Maximum Dose
|
|---|---|---|
| adults and children 12 years of age and older
| 2 tablets once a day preferably at bedtime; increase as needed, or as directed by a doctor
| 4 tablets in the morning and 4 tablets at bedtime
|
| children under 12 years
| ask a doctor
|
Other information
- each tablet contains: calcium 20 mg, sodium 6 mg
- store at room temperature 15° - 30°C (59°-86°F)
| SENNA-S
sennosides and docusate sodium tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Chain Drug Consortium, LLC (101668460) |
| Registrant - Geri-Care Pharmaceutical Corp (611196254) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Geri-Care Pharmaceutical Corp | 611196254 | repack(68016-746) | |
Revised: 12/2021
Document Id: d22f1cff-1135-97b0-e053-2995a90a1b87
Set id: 61f84b81-99fb-626d-e053-2a91aa0a8b83
Version: 3
Effective Time: 20211202
Chain Drug Consortium, LLC