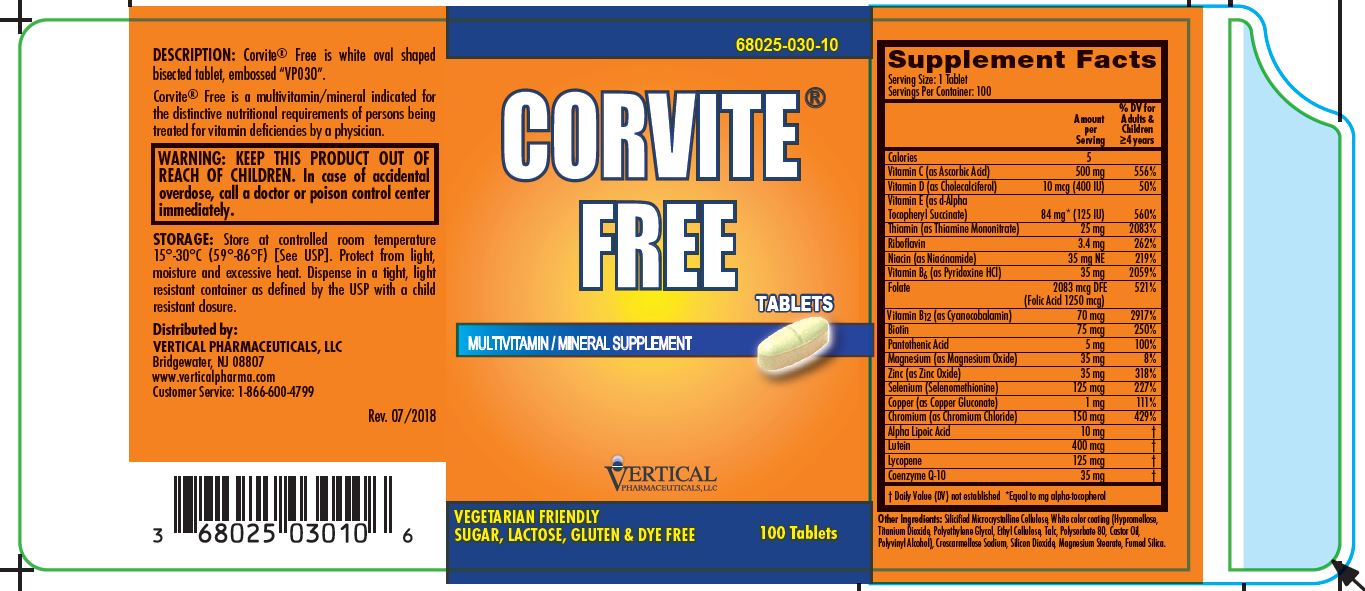
CORVITE FREE- ascorbic acid, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, pantothenic acid, magnesium oxide, zinc oxide, selenomethionine, copper gluconate, chromic chloride, .alpha.-lipoic acid, lutein, lycopene, and ubidecarenone tablet, coated
Vertical Pharmaceuticals, LLC
----------
Corvite® Free
MULTIVITAMIN/MINERAL SUPPLEMENT
SUPPLEMENT FACTS
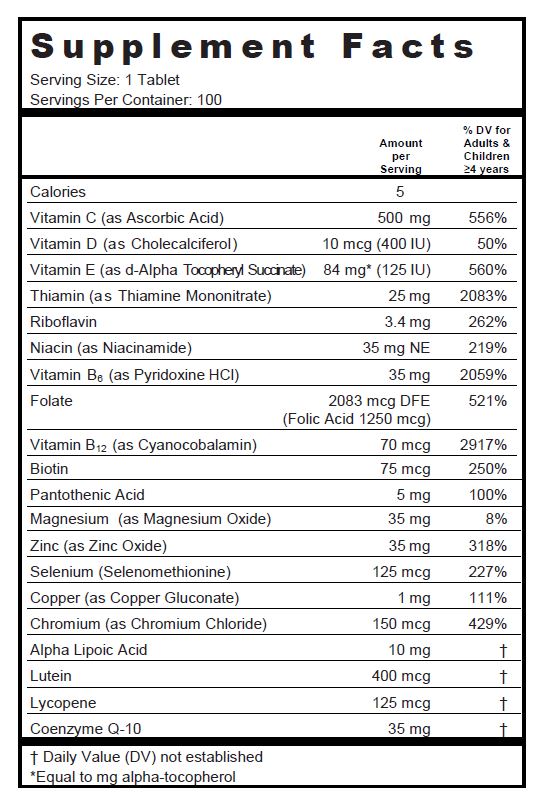
Other Ingredients: Silicified Microcrystalline Cellulose, White color coating (Hypromellose, Titanium Dioxide, Polyethylene Glycol, Ethyl Cellulose, Talc, Polysorbate 80, Castor Oil, Polyvinyl Alcohol), Croscarmellose Sodium, Silicon Dioxide, Magnesium Stearate, Fumed Silica.
VEGETARIAN FRIENDLY
SUGAR- LACTOSE- GLUTEN- AND DYE-FREE
Corvite® Free is a multivitamin/mineral indicated for the distinctive nutritional requirements of persons being treated for vitamin deficiencies by a physician.
CONTRAINDICATIONS
Corvite® Free should not be used by patients with a known hypersensitivity to any of the listed ingredients.
WARNINGS
| WARNING: KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
PRECAUTIONS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including cardiovascular diagnostic tests (e.g. troponin) and hormone tests, and may lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
DRUG INTERACTIONS
Corvite® Free tablets are not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained herein. However, allergic and idiosyncratic reactions are possible at lower levels.
HOW SUPPLIED
Corvite® Free Multivitamin/Mineral is supplied in bottles of 100 tablets.
Product Code: 68025-030-10
STORAGE
Store at controlled room temperature 15°-30°C (59°-86°F) [See USP]. Protect from light, moisture and excessive heat. Dispense in a tight, light resistant container as defined by the USP with a child resistant closure.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Vertical Pharmaceuticals at 1-877-95-VERTI (1-877-958-3784) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Customer Service: 1-866-600-4799 Rev. 07/2018
Distributed by:
Vertical Pharmaceuticals, LLC
Bridgewater, NJ 08807

| CORVITE FREE
ascorbic acid, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, pantothenic acid, magnesium oxide, zinc oxide, selenomethionine, copper gluconate, chromic chloride, .alpha.-lipoic acid, lutein, lycopene, and ubidecarenone tablet, coated |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| imprint | ||
| scoring | 2 | |
| shape | ||
| size (solid drugs) | 19 mm | |
| Labeler - Vertical Pharmaceuticals, LLC (173169017) |