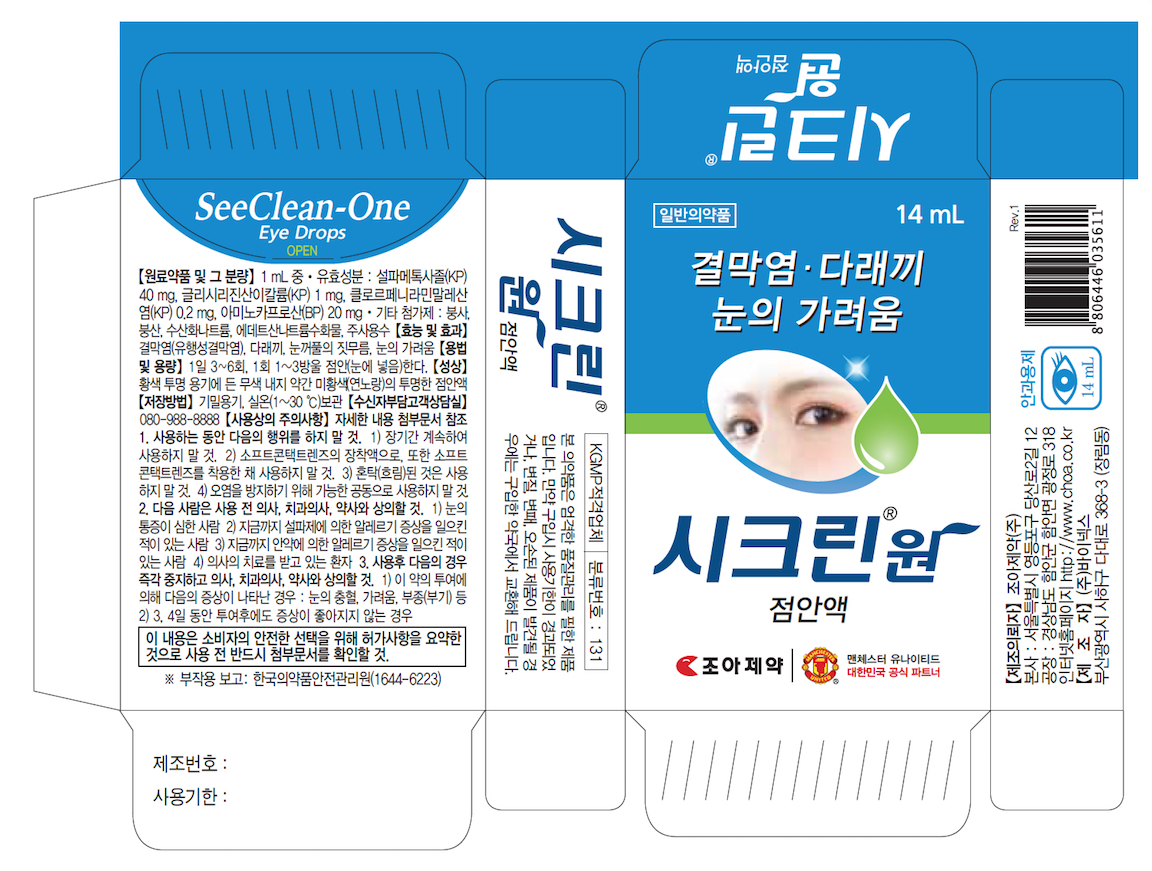
SEE CLEAN-ONE EYE DROPS- boric acid liquid
Cho-A Pharm.Co.,Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
See Clean-One Eye Drops
Uses
For the temporarily relief of
- burning and irritation due to dryness of the eye
- redness of the eye due to minor eye irritation
Warnings
For external use only
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before using
- replace cap after use
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- conditions worsen
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
| SEE CLEAN-ONE EYE DROPS
boric acid liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cho-A Pharm.Co.,Ltd. (688056831) |
| Registrant - Cho-A Pharm.Co.,Ltd. (688056831) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cho-A Pharm.Co.,Ltd. | 688056831 | manufacture(58354-105) | |