KAYEXALATE- sodium polystyrene sulfonate powder, for suspension
sanofi-aventis U.S. LLC
----------
DESCRIPTION
KAYEXALATE, brand of sodium polystyrene sulfonate is a benzene, diethenyl-polymer, with ethenylbenzene, sulfonated, sodium salt and has the following structural formula:
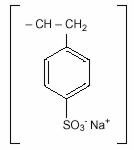
The drug is a cream to light brown finely ground, powdered form of sodium polystyrene sulfonate, a cation-exchange resin prepared in the sodium phase with an in vitro exchange capacity of approximately 3.1 mEq (in vivo approximately 1 mEq) of potassium per gram. The sodium content is approximately 100 mg (4.1 mEq) per gram of the drug. It can be administered orally or in an enema.
CLINICAL PHARMACOLOGY
As the resin passes along the intestine or is retained in the colon after administration by enema, the sodium ions are partially released and are replaced by potassium ions. For the most part, this action occurs in the large intestine, which excretes potassium ions to a greater degree than does the small intestine. The efficiency of this process is limited and unpredictably variable. It commonly approximates the order of 33 percent but the range is so large that definitive indices of electrolyte balance must be clearly monitored.
Metabolic data are unavailable.
CONTRAINDICATIONS
KAYEXALATE is contraindicated in the following conditions: patients with hypokalemia, patients with a history of hypersensitivity to polystyrene sulfonate resins, obstructive bowel disease, neonates with reduced gut motility (postoperatively or drug induced) and oral administration in neonates (see PRECAUTIONS).
WARNINGS
Intestinal Necrosis
Cases of intestinal necrosis, which may be fatal, and other serious gastrointestinal adverse events (bleeding, ischemic colitis, perforation) have been reported in association with KAYEXALATE use. The majority of these cases reported the concomitant use of sorbitol. Risk factors for gastrointestinal adverse events were present in many of the cases including prematurity, history of intestinal disease or surgery, hypovolemia, and renal insufficiency and failure. Concomitant administration of sorbitol is not recommended (see PRECAUTIONS, Drug Interactions).
- Use only in patients who have normal bowel function. Avoid use in patients who have not had a bowel movement post-surgery.
- Avoid use in patients who are at risk for developing constipation or impaction (including those with history of impaction, chronic constipation, inflammatory bowel disease, ischemic colitis, vascular intestinal atherosclerosis, previous bowel resection, or bowel obstruction).
- Discontinue use in patients who develop constipation.
Alternative Therapy in Severe Hyperkalemia
Since effective lowering of serum potassium with KAYEXALATE may take hours to days, treatment with this drug alone may be insufficient to rapidly correct severe hyperkalemia associated with states of rapid tissue breakdown (e.g., burns and renal failure) or hyperkalemia so marked as to constitute a medical emergency. Therefore, other definitive measures, including dialysis, should always be considered and may be imperative.
Hypokalemia
Serious potassium deficiency can occur from therapy with KAYEXALATE. The effect must be carefully controlled by frequent serum potassium determinations within each 24 hour period. Since intracellular potassium deficiency is not always reflected by serum potassium levels, the level at which treatment with KAYEXALATE should be discontinued must be determined individually for each patient. Important aids in making this determination are the patient's clinical condition and electrocardiogram. Early clinical signs of severe hypokalemia include a pattern of irritable confusion and delayed thought processes.
Electrocardiographically, severe hypokalemia is often associated with a lengthened Q-T interval, widening, flattening, or inversion of the T wave, and prominent U waves. Also, cardiac arrhythmias may occur, such as premature atrial, nodal, and ventricular contractions, and supraventricular and ventricular tachycardias. The toxic effects of digitalis are likely to be exaggerated. Marked hypokalemia can also be manifested by severe muscle weakness, at times extending into frank paralysis.
Electrolyte Disturbances
Like all cation-exchange resins, KAYEXALATE is not totally selective (for potassium) in its actions, and small amounts of other cations such as magnesium and calcium can also be lost during treatment. Accordingly, patients receiving KAYEXALATE should be monitored for all applicable electrolyte disturbances.
Systemic Alkalosis
Systemic alkalosis has been reported after cation-exchange resins were administered orally in combination with nonabsorbable cation-donating antacids and laxatives such as magnesium hydroxide and aluminum carbonate. Magnesium hydroxide should not be administered with KAYEXALATE. One case of grand mal seizure has been reported in a patient with chronic hypocalcemia of renal failure who was given KAYEXALATE with magnesium hydroxide as laxative. (See PRECAUTIONS, Drug Interactions.)
PRECAUTIONS
Caution is advised when KAYEXALATE is administered to patients who cannot tolerate even a small increase in sodium loads (i.e., severe congestive heart failure, severe hypertension, or marked edema). In such instances compensatory restriction of sodium intake from other sources may be indicated.
In the event of clinically significant constipation, treatment with KAYEXALATE should be discontinued until normal bowel motion is resumed (see WARNINGS, Intestinal Necrosis).
Drug Interactions
Antacids
The simultaneous oral administration of KAYEXALATE with nonabsorbable cation-donating antacids and laxatives may reduce the resin's potassium exchange capability.
Non-absorbable cation-donating antacids and laxatives
Systemic alkalosis has been reported after cation-exchange resins were administered orally in combination with nonabsorbable cation-donating antacids and laxatives such as magnesium hydroxide and aluminum carbonate. Magnesium hydroxide should not be administered with KAYEXALATE. One case of grand mal seizure has been reported in a patient with chronic hypocalcemia of renal failure who was given KAYEXALATE with magnesium hydroxide as a laxative.
Intestinal obstruction due to concretions of aluminum hydroxide when used in combination with KAYEXALATE has been reported.
Digitalis
The toxic effects of digitalis on the heart, especially various ventricular arrhythmias and A-V nodal dissociation, are likely to be exaggerated by hypokalemia, even in the face of serum digoxin concentrations in the "normal range". (See WARNINGS.)
Sorbitol
Concomitant use of Sorbitol with KAYEXALATE has been implicated in cases of intestinal necrosis, which may be fatal. Therefore, concomitant administration is not recommended. (See WARNINGS.)
Pregnancy Category C
Animal reproduction studies have not been conducted with KAYEXALATE. It is also not known whether KAYEXALATE can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. KAYEXALATE should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when KAYEXALATE is administered to a nursing woman.
Pediatric Use
The effectiveness of KAYEXALATE in pediatric patients has not been established. In neonates, KAYEXALATE should not be given by the oral route. In both children and neonates particular care should be observed with rectal administration, as excessive dosage or inadequate dilution could result in impaction of the resin.
Due to the risk of digestive hemorrhage or intestinal necrosis, particular care should be observed in premature infants or low birth weight infants.
ADVERSE REACTIONS
KAYEXALATE may cause some degree of gastric irritation. Anorexia, nausea, vomiting, and constipation may occur especially if high doses are given. Also, hypokalemia, hypocalcemia, hypomagnesemia and significant sodium retention, and their related clinical manifestations, may occur (see WARNINGS). Occasionally diarrhea develops. Large doses in elderly individuals may cause fecal impaction (see PRECAUTIONS). Rare instances of intestinal necrosis have been reported. Intestinal obstruction due to concretions of aluminum hydroxide, when used in combination with KAYEXALATE, has been reported.
The following events have been reported from worldwide post marketing experience:
- Fecal impaction following rectal administration, particularly in children;
- Gastrointestinal concretions (bezoars) following oral administration;
- Ischemic colitis, gastrointestinal tract ulceration or necrosis which could lead to intestinal perforation; and,
- Rare cases of acute bronchitis and/or broncho-pneumonia associated with inhalation of particles of polystyrene sulfonate.
OVERDOSAGE
Overdosage may result in electrolyte disturbances including hypokalemia, hypocalcemia, and hypomagnesemia. Biochemical disturbances resulting from overdosage may give rise to clinical signs and symptoms of hypokalemia, including: irritability, confusion, delayed thought processes, muscle weakness, hyporeflexia, which may progress to frank paralysis and/or apnea. Tetany may occur. Electrocardiographic changes may be consistent with hypokalemia or hypocalcemia; cardiac arrhythmias may occur. Appropriate measures should be taken to correct serum electrolytes (potassium, calcium, magnesium), and the resin should be removed from the alimentary tract by appropriate use of laxatives or enemas.
DOSAGE AND ADMINISTRATION
Suspension of this drug should be freshly prepared and not stored beyond 24 hours.
The average daily adult dose of the resin is 15 g to 60 g. This is best provided by administering 15 g (approximately 4 level teaspoons) of KAYEXALATE one to four times daily. One gram of KAYEXALATE contains 4.1 mEq of sodium; one level teaspoon contains approximately 3.5 g of KAYEXALATE and 15 mEq of sodium. (A heaping teaspoon may contain as much as 10 g to 12 g of KAYEXALATE.) Since the in vivo efficiency of sodium-potassium exchange resins is approximately 33 percent, about one third of the resin's actual sodium content is being delivered to the body.
In smaller children and infants, lower doses should be employed by using as a guide a rate of 1 mEq of potassium per gram of resin as the basis for calculation.
Each dose should be given as a suspension in a small quantity of water or, for greater palatability, in syrup. The amount of fluid usually ranges from 20 mL to 100 mL, depending on the dose, or may be simply determined by allowing 3 mL to 4 mL per gram of resin. Healthcare professionals should follow full aspiration precautions when administering this product, such as placing and maintaining the patient in an upright position while the resin is being administered.
The resin may be introduced into the stomach through a plastic tube and, if desired, mixed with a diet appropriate for a patient in renal failure.
The resin may also be given, although with less effective results, in an enema consisting (for adults) of 30 g to 50 g every six hours. Each dose is administered as a warm emulsion (at body temperature) in 100 mL of aqueous vehicle. The emulsion should be agitated gently during administration. The enema should be retained as long as possible and followed by a cleansing enema.
After an initial cleansing enema, a soft, large size (French 28) rubber tube is inserted into the rectum for a distance of about 20 cm, with the tip well into the sigmoid colon, and taped in place. The resin is then suspended in the appropriate amount of aqueous vehicle at body temperature and introduced by gravity, while the particles are kept in suspension by stirring. The suspension is flushed with 50 mL or 100 mL of fluid, following which the tube is clamped and left in place. If back leakage occurs, the hips are elevated on pillows or a knee-chest position is taken temporarily. A somewhat thicker suspension may be used, but care should be taken that no paste is formed, because the latter has a greatly reduced exchange surface and will be particularly ineffective if deposited in the rectal ampulla. The suspension is kept in the sigmoid colon for several hours, if possible. Then, the colon is irrigated with nonsodium containing solution at body temperature in order to remove the resin. Two quarts of flushing solution may be necessary. The returns are drained constantly through a Y tube connection. While the use of sorbitol is not recommended, particular attention should be paid to this cleansing enema if sorbitol has been used.
The intensity and duration of therapy depend upon the severity and resistance of hyperkalemia.
KAYEXALATE should not be heated for to do so may alter the exchange properties of the resin.
HOW SUPPLIED
KAYEXALATE is available as a cream to light brown, finely ground powder in jars of 1 pound (453.6 g), NDC 0024-1075-01.
| KAYEXALATE
sodium polystyrene sulfonate powder, for suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Purolite S.R.L. | 565718892 | MANUFACTURE(0024-1075), ANALYSIS(0024-1075) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Valeant Phramaceuticals International, Inc. | 248902996 | ANALYSIS(0024-1075), LABEL(0024-1075), PACK(0024-1075) | |
