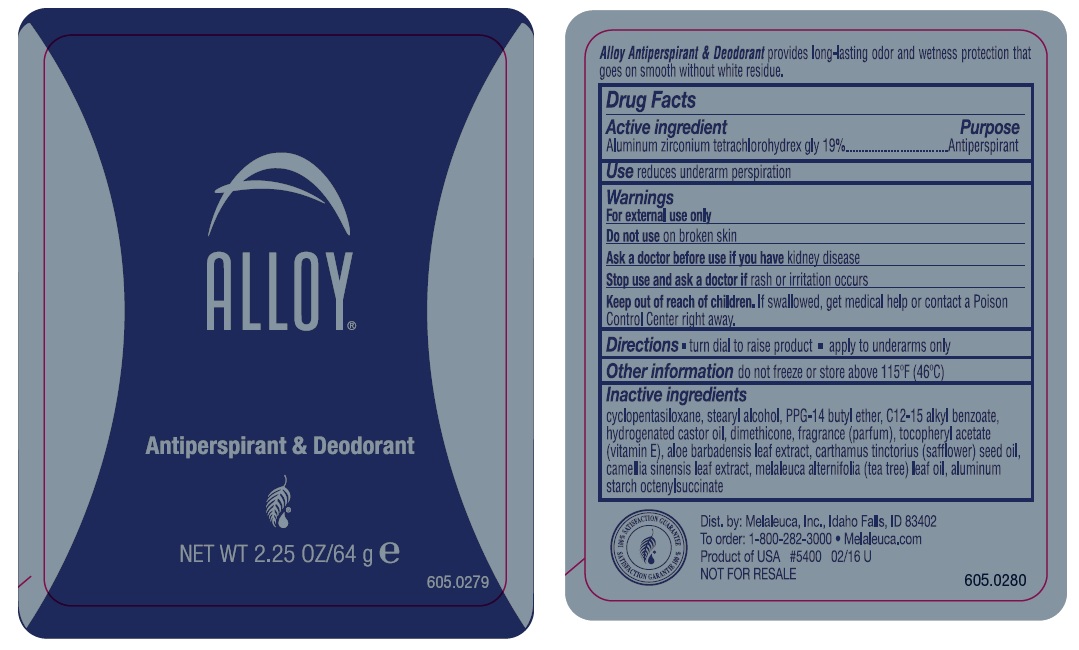
MELALEUCA ALLOY ORIGINAL- aluminum zirconium tetrachlorohydrex gly stick
VVF Kansas Services LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Melaleuca Alloy Original
Keep out of reach of children.
If swallowed, get medical help or contact a Poison control Center right away.
Inactive ingredients
cyclopentasiloxane, stearyl alcohol, PPG-14 butyl ether, C12-15 alkyl benzoate,
hydrogenated castor oil, dimethicone, fragrance (parfum), tocopheryl acetate
(vitamin E), aloe barbadensis leaf extract, carthamus tinctorius (safflower) seed oil,
camellia sinensis leaf extract, melaleuca alternifolia (tea tree) leaf oil,
aluminum starch octenylsuccinate
| MELALEUCA ALLOY ORIGINAL
aluminum zirconium tetrachlorohydrex gly stick |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - VVF Kansas Services LLC (833164242) |
| Registrant - VVF Kansas Services LLC (833164242) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| VVF Kansas Services LLC | 833164242 | manufacture(10889-421) | |
Revised: 11/2018
Document Id: 7a8e3330-b59a-2026-e053-2a91aa0a9c23
Set id: 60f2318c-f9b7-19ff-e053-2a91aa0a84cf
Version: 2
Effective Time: 20181113
VVF Kansas Services LLC