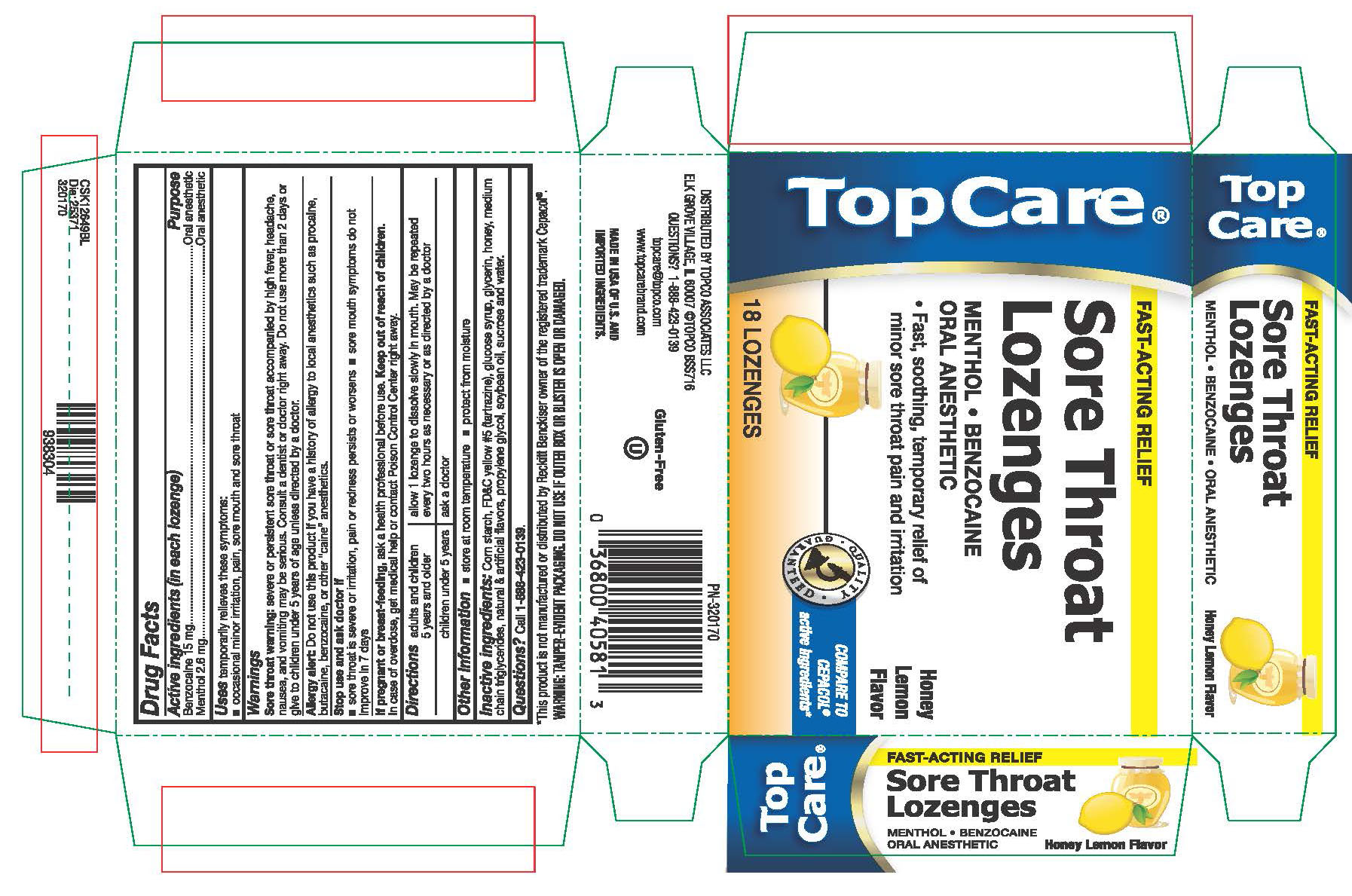
HONEY LEMON SORE THROAT LOZENGES- benzocaine lozenge
TopCo
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Benzocaine 15 mg
Menthol 2.6 mg
Uses
Temporarily relieves the following symptoms:
occasional minor irritation, pain, sore mouth and sore throat
Warnings
Sore throat warning: Severe or persistent sore throat or sore throat that is accompanied by fever, headache, nausea, and vomiting, may be serious. Consult a dentist or doctor right away. Do not use more than 2 days or give to children under 5 years of age unless directed by a doctor.
Allergy Alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics.
Stop use and consult doctor if
sore throat is severe, or irritation, pain or redness persists or worsens
sore mouth symptoms do not improve in 7 days
Directions
adults and children 5 years and older - allow 1 lozenge to dissolve slowly in mouth. May be repeated every 2 hours as necessary or as directed by a dentist or doctor.
children under 5 years - ask a doctor
| HONEY LEMON SORE THROAT LOZENGES
benzocaine lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| Labeler - TopCo (006935977) |
| Registrant - Bestco Inc. (002149136) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bestco Inc. | 002149136 | manufacture(36800-833) | |