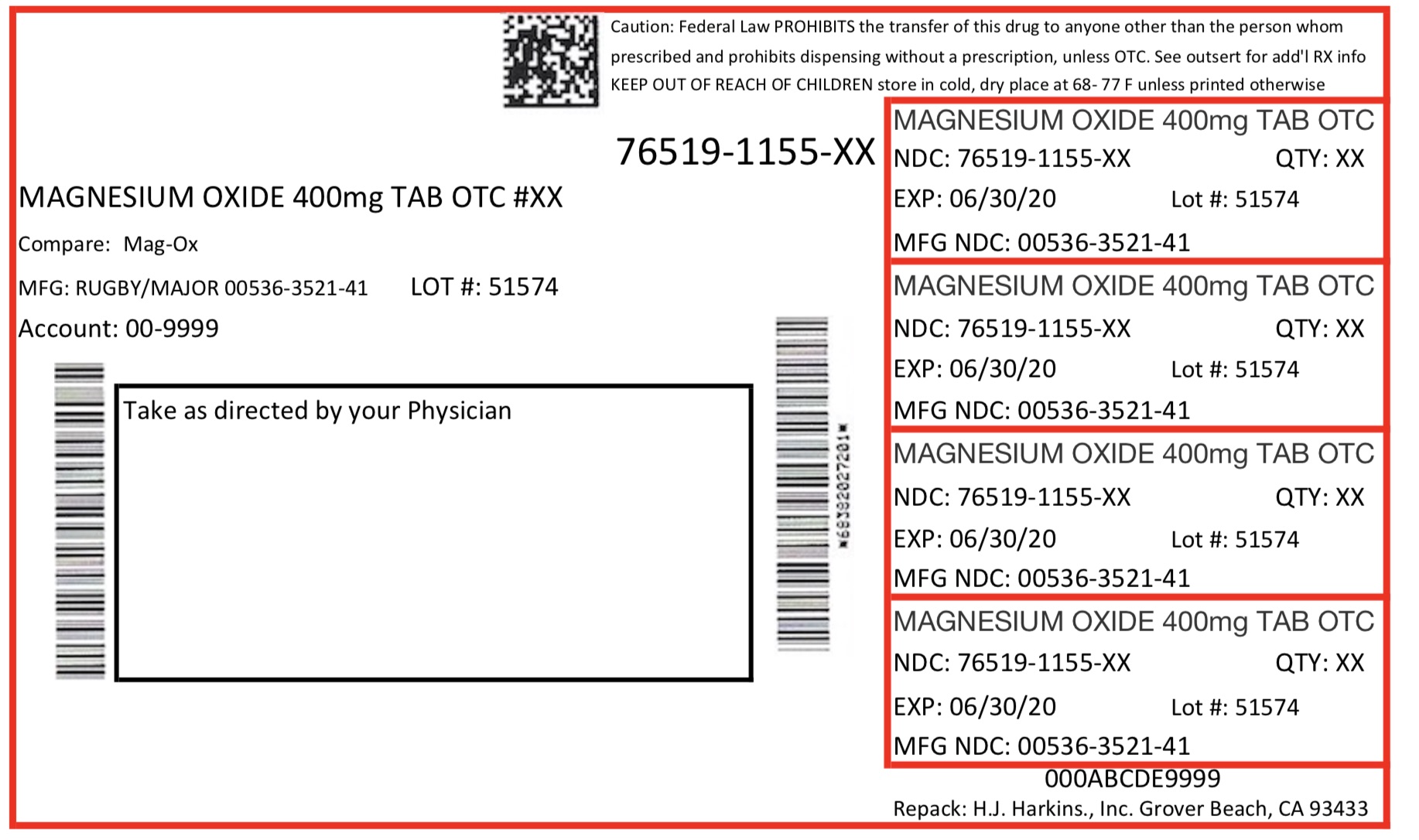
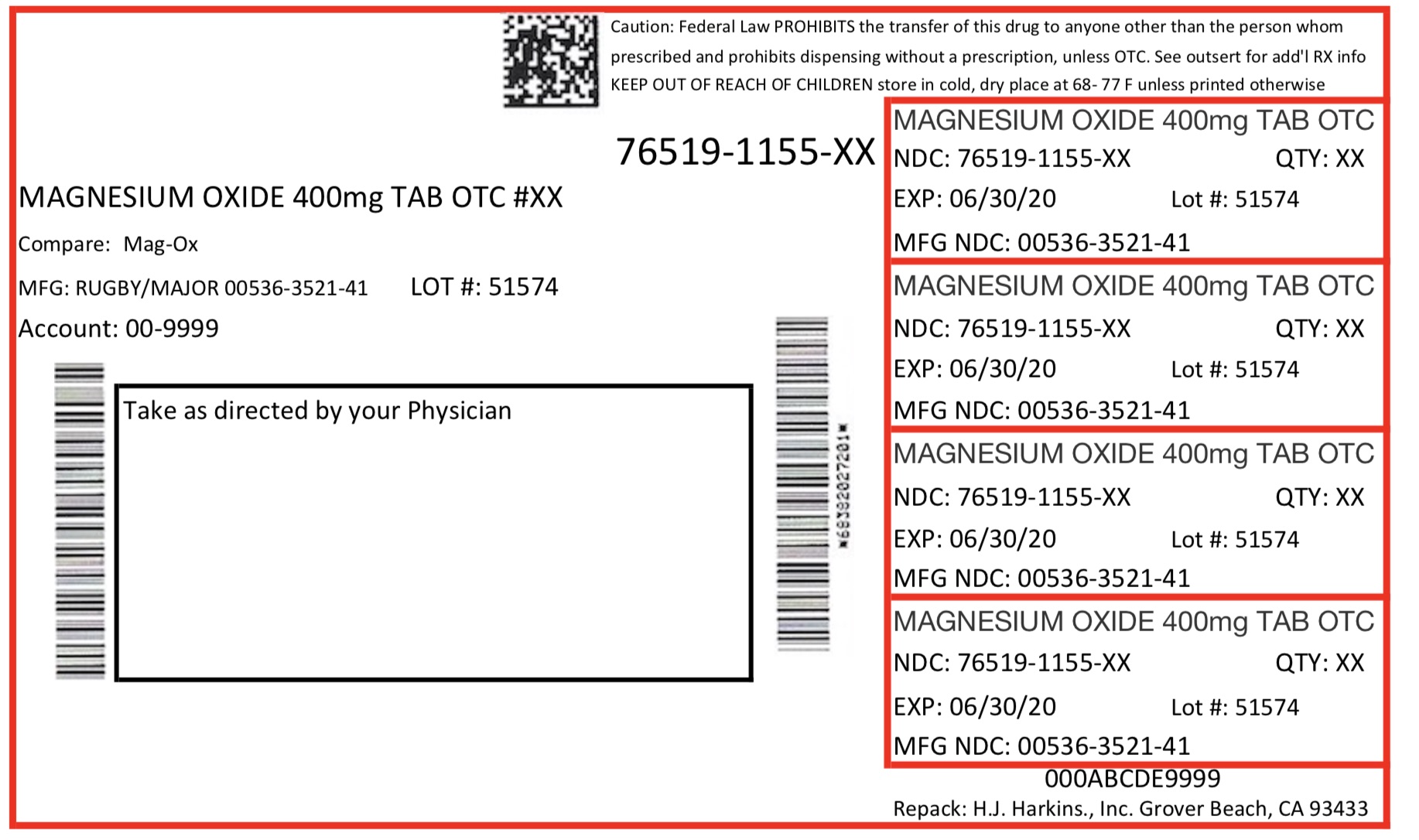
Label: MAGNESIUM OXIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 76519-1155-6 - Packager: H. J. Harkins Company Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 19, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- KEEP OUT OF REACH OF CHILDREN
- Dosage and Administration
- Storage & Handling
- Inactive Ingredients
- Indications & Usage
-
Warnings
Do not take this product if you are presently taking a prescription drug without consulting your physician or other health care professional. If you have kidney disease, take only under the supervision of a physician. May have a laxative effect. If pregnant or breast-feeding, ask a health professional before use.
Pregnancy or Breast Feeding
If pregnant or breast-feeding, ask a health professional before use.
Keep Out of Reach of Children
- Package Label. Prinicpal Display Panel
-
INGREDIENTS AND APPEARANCE
MAGNESIUM OXIDE
magnesium oxide tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76519-1155 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 400 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white Score score with uneven pieces Shape ROUND Size 11mm Flavor Imprint Code T Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76519-1155-6 60 in 1 CONTAINER; Type 0: Not a Combination Product 06/16/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 06/16/2017 Labeler - H. J. Harkins Company Inc. (147681894) Establishment Name Address ID/FEI Business Operations H. J. Harkins Company Inc. 147681894 manufacture(76519-1155) , relabel(76519-1155) , repack(76519-1155)