Label: SHORT AND GIANT RAGWEED POLLEN MIX- ambrosia artemisiifolia and ambrosia trifida solution
SHORT RAGWEED POLLEN- ambrosia artemisiifolia solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 22840-0300-2, 22840-0300-4, 22840-0300-5, 22840-0301-2, view more22840-0301-4, 22840-0301-5 - Packager: Greer Laboratories, Inc.
- Category: NON-STANDARDIZED ALLERGENIC LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract safely and effectively. See full prescribing information for Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract.
Short Ragweed Pollen Allergenic Extract
Short and Giant Ragweed Pollen Mix Allergenic Extract
Solutions for percutaneous, intradermal, or subcutaneous administration.
Initial U.S. Approval: 1982
WARNING: SEVERE ALLERGIC REACTIONS
See full prescribing information for complete boxed warning.
- Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract can cause severe life-threatening systemic reactions, including anaphylaxis. (5.1)
- Do not administer these products to patients with severe, unstable or uncontrolled asthma. (4)
- Observe patients in the office for at least 30 minutes following treatment.Emergency measures and personnel trained in their use must be available immediately in the event of a life-threatening reaction. (5.1)
- Patients with extreme sensitivity to these products, on an accelerated immunotherapy build-up, switching to another lot, receiving high doses of these products, or those also exposed to similar allergens may be at increased risk of a severe allergic reaction. (5.1)
- These products may not be suitable for patients with certain underlying medical conditions that may reduce their ability to survive a serious allergic reaction, or for patients who may be unresponsive to epinephrine or inhaled bronchodilators, such as those taking beta-blockers. (5.1, 5.2)
INDICATIONS AND USAGE
Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract are indicated for:
- Skin test diagnosis of patients with a clinical history of allergy to ragweed pollen (Short Ragweed or Short and Giant Ragweed pollen). (1)
- Immunotherapy for the reduction of ragweed pollen-induced allergic symptoms confirmed by positive skin test or by in vitro testing for pollen-specific IgE antibodies for Short and/or Giant Ragweed pollen. (1)
DOSAGE AND ADMINISTRATION
For percutaneous, intradermal or subcutaneous use only.
The extracts are diluted with sterile diluents when used for intradermal testing or subcutaneous immunotherapy. For percutaneous testing, the extracts are diluted with sterile diluents in patients expected to be at greater risk for systemic allergic reaction. Dosages vary by mode of administration and by individual response. See full prescribing information for instructions on preparation, administration, and adjustments of dose. (2.1)
DOSAGE FORMS AND STRENGTHS
Short Ragweed Pollen Allergenic Extract solution; stock concentrates at 1:20 weight/volume with Antigen E (Amb a 1) concentration labeled in Units/milliliter. (3)
Short and Giant Ragweed Pollen Mix Allergenic Extract solution: stock concentrates at 1:20 weight/volume with Antigen E (Amb a 1) concentration labeled in Units/milliliter. (3)
CONTRAINDICATIONS
- Severe, unstable or uncontrolled asthma. (4)
- History of any severe systemic allergic reaction or any severe local reaction to subcutaneous allergen immunotherapy. (4)
WARNINGS AND PRECAUTIONS
Severe allergic reactions have occurred following the administration of other allergenic extracts and may occur in individuals following the administration of Short Ragweed Pollen Allergenic Extract or Short and Giant Ragweed Pollen Mix Allergenic Extract in the following situations:
- Extreme sensitivity to these products, receipt of high doses of these products, or concomitant exposure to similar environmental allergens. (5.1)
- Receiving an accelerated immunotherapy build-up schedule (e.g., “rush” immunotherapy), or changing from one allergenic lot to another. (5.1)
ADVERSE REACTIONS
The most common adverse reactions occurring in 26 to 82% of all patients who receive subcutaneous immunotherapy are local adverse reactions at the injection site (e.g., erythema, itching, swelling, tenderness, pain). (6)
Systemic adverse reactions, occurring in < 7% of patients who receive subcutaneous immunotherapy, include generalized skin erythema, urticaria, pruritus, angioedema, rhinitis, wheezing, laryngeal edema, and hypotension. These can be fatal. (6)
To report SUSPECTED ADVERSE REACTIONS, contact GREER Laboratories, Inc. at 1-855-274-1322 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Antihistamines and other medications that suppress histamine, including topical corticosteroids, topical anesthetics and tricyclic antidepressants can interfere with skin test results. (7.1)
See 17 for PATIENT COUNSELING INFORMATION
-
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SEVERE ALLERGIC REACTIONS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Administration
2.2 Diagnostic Testing
2.3 Immunotherapy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Systemic Adverse Reactions
5.2 Epinephrine
5.3 Cross-Reactions and Dose Sensitivity
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Antihistamines
7.2 Topical Corticosteroids and Topical Anesthetics
7.3 Tricyclic Antidepressants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
*Sections or subsections omitted from the Full Prescribing Information are not listed. -
BOXED WARNING
(What is this?)
FULL PRESCRIBING INFORMATION
WARNING: SEVERE ALLERGIC REACTIONS
- Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract can cause severe life-threatening systemic reactions, including anaphylaxis. (5.1)
- Do not administer these products to patients with severe, unstable or uncontrolled asthma. (4)
- Observe patients in the office for at least 30 minutes following treatment.Emergency measures and personnel trained in their use must be available immediately in the event of a life-threatening reaction. (5.1)
- Patients with extreme sensitivity to these products, those on an accelerated immunotherapy build-up schedule, those switching to another allergenic lot, those receiving high doses of these products, or those also exposed to similar allergens may be at increased risk of a severe allergic reaction. (5.1)
- These products may not be suitable for patients with certain underlying medical conditions that may reduce their ability to survive a serious allergic reaction. (5.1)
- These products may not be suitable for patients who may be unresponsive to epinephrine or inhaled bronchodilators, such as those taking beta-blockers. (5.2)
-
1 INDICATIONS AND USAGE
Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract are indicated for:
- Skin test diagnosis of patients with a clinical history of allergy to ragweed pollen (Short Ragweed or Short and Giant Ragweed Pollen).
- Immunotherapy for the reduction of ragweed pollen-induced allergic symptoms confirmed by positive skin test or by in vitro testing for pollen-specific IgE antibodies for Short and/or Giant Ragweed pollen.
-
2 DOSAGE AND ADMINISTRATION
For percutaneous, intradermal or subcutaneous use only.
The extracts are diluted with sterile diluents when used for intradermal testing or subcutaneous immunotherapy. For percutaneous testing, the extracts are diluted with sterile diluents in patients expected to be at greater risk for systemic allergic reaction. Dosages vary by mode of administration and by individual response.
2.1 Preparation for Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard solution if either of these conditions exist.
The extracts are diluted with sterile diluents when used for percutaneous and intradermal testing, or for subcutaneous immunotherapy.
Undiluted 1:20 weight/volume stock concentrate is used for percutaneous testing. To prepare 10-fold dilutions for percutaneous testing in patients suspected to be at greater risk for systemic allergic reaction, start with a 1:20 weight/volume stock concentrate. Proceed as in Table 1. The 10-fold dilution series uses 0.5 milliliters of concentrate added to 4.5 milliliters of sterile 50% glycerin diluent. Subsequent dilutions are made in a similar manner.
To prepare 10-fold dilutions for intradermal testing and immunotherapy, start with a 1:20 weight/volume stock concentrate. Proceed as in Table 1. The 10-fold dilution series uses 0.5 milliliter of concentrate added to 4.5 milliliters of sterile diluent (glycerin-free diluents for intradermal testing, glycerin-free or 10% glycerin saline diluents for immunotherapy). Subsequent dilutions are made in a similar manner.
Table 1: 10-fold Dilution Series Dilution Extract Milliliters of Diluent Dilution Strength Dilution Strength [AgE (Amb a 1) Units/mL]
Short Ragweed Pollen Short and Giant Ragweed Pollen Mix 0 Concentrate 1:20 150 - 300 75 - 150 1 0.5 mL Concentrate 4.5 1:200 15 - 30 7.5 - 15 2 0.5 mL Dilution 1 4.5 1:2,000 1.5 - 3 0.75 - 1.5 3 0.5 mL Dilution 2 4.5 1:20,000 0.15 - 0.3
0.075 - 0.15 4 0.5 mL Dilution 3 4.5 1:200,000 0.015 - 0.03
0.0075 - 0.015
5 0.5 mL Dilution 4 4.5 1:2,000,000 0.0015 - 0.003 0.00075 - 0.0015 6 0.5 mL Dilution 5 4.5 1:20,000,000 0.00015 - 0.0003 0.000075 - 0.00015 Undiluted 1:20 weight/volume stock concentrate is typically used for percutaneous testing. To prepare 5-fold dilutions for percutaneous testing in patients suspected to be at greater risk for systemic allergic reaction, start with a 1:20 weight/volume stock concentrate. Proceed as in Table 2. The 5-fold dilution series uses 1 milliliter of concentrate added to 4 milliliters of sterile 50% glycerin diluent. Subsequent dilutions are made in a similar manner.
To prepare 5-fold dilutions for intradermal testing or immunotherapy, start with a 1:20 weight/volume (w/v) stock concentrate. Proceed as in Table 2. The 5-fold dilution series uses 1 milliliter of concentrate added to 4 milliliters of sterile diluent (glycerin-free diluents for intradermal testing, glycerin-free or 10% glycerin saline diluents for immunotherapy). Subsequent dilutions are made in a similar manner.
Table 2: 5-fold Dilution Series Dilution Extract Milliliters of Diluent Dilution Strength (w/v) Dilution Strength [AgE (Amb a 1) Units/mL] Short Ragweed Pollen Short and Giant Ragweed Pollen Mix 0 Concentrate 1:20 150 - 300 75 - 150 1 1 mL Concentrate 4 1:100 30 - 60 15 - 30 2 1 mL Dilution 1 4 1:500 6 - 12 3 - 6 3 1 mL Dilution 2 4 1:2,500 1.2 - 2.4 0.06 - 1.2 4 1 mL Dilution 3 4 1:12,500 0.24 - 0.48 0.12 - 0.24
5 1 mL Dilution 5 4 1:62,500 0.048 - 0.096 0.024 - 0.048 6 1 mL Dilution 4 4 1:312,500 0.0096 - 0.0192
0.0048 - 0.0096 2.2 Diagnostic Testing
Diagnostic testing can be performed via percutaneous or intradermal administration of the Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract. A positive skin test reaction must be interpreted in relation to the patient’s history and known exposure to the allergen.
If a skin test with the Short and Giant Ragweed Pollen Mix Allergenic Extract elicits a positive reaction, then the single-species ragweed pollen allergenic extracts can be used to determine the degree of sensitivity to each, and to guide in the selection of extracts and their concentration for immunotherapy, if indicated.
Percutaneous Skin Testing
Determine the patient’s sensitivity to the ragweed pollen allergens.
Prick or puncture testing: use 1:20 weight/volume extract stock concentrate.
In patients suspected to be at greater risk for systemic allergic reaction, initiate testing with serial 10-fold or 5-fold dilutions.
Preparation and Dose
For percutaneous testing (prick or puncture) use 1:20 weight/volume stock concentrate. If a lower concentration is desired in patients suspected to be at greater risk for a systemic allergic reaction, 10-fold or 5-fold dilutions of the concentrate can be tested.
Prick test: Place one drop of extract or control on the skin and, with a skin test device, pierce through the drop into the skin with a slight lifting motion. Alternatively, use skin test devices loaded with the extract from the storage trays in a similar manner, or in accordance with the device manufacturer’s recommendations.
Puncture test: Place one drop of extract or control on the skin and pierce the skin through the drop with a skin test device perpendicular to the skin. Alternatively, use skin test devices loaded with the extract from the storage trays in a similar manner, or in accordance with the device manufacturer’s recommendations.
Interpreting Results
When using percutaneous skin test devices, follow the directions provided with the test devices. A glycerinated histamine control solution (6 milligrams/milliliter or 1 milligram/milliliter histamine base) may be used as the positive control. A 50% glycerin saline solution may be used as the negative control.
Read and record skin test responses 15 to 20 minutes after exposure. Individual patient reactivity can vary with time, allergen potency, and/or immunotherapy, as well as testing technique. The most reliable method of recording a skin test reaction is to measure the largest diameter of both wheal and erythema. While some correlation exists between the size of the skin test reaction and the degree of sensitivity, other factors should be considered in the diagnosis of allergy to specific allergens (see Figure 1 below).
Figure 1: Measurement of Wheal and Flare
To measure the Wheal and Flare, use a paper or plastic millimeter skin reaction guide as shown below.
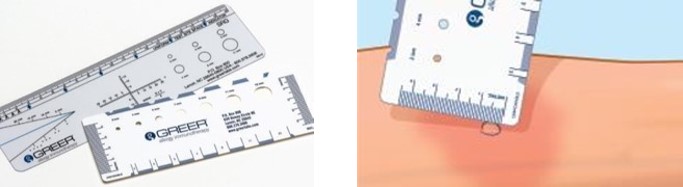
When the timer goes off 15 minutes after application of the skin test, measure the length and midpoint orthogonal width of each flare and wheal from the inner edge of the reaction.

The length of the skin test is defined as the largest diameter and the width of the skin test is defined as the diameter perpendicular to the length at its midpoint. Consider the wheal and flare as separate entities. First measure the flare and then independently measure the wheal.
Measuring the Flare
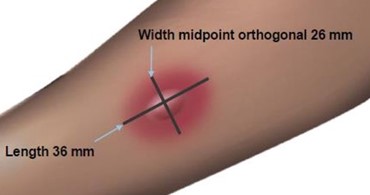
Measuring the Wheal
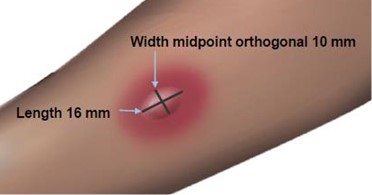
The average diameter measurement in the example above of the flare is (26 mm + 36 mm)/2 = 31 mm and the average diameter of the wheal is (10 mm + 16 mm)/2 = 13 mm.
Responses to positive controls should be at least 3 millimeters larger than responses to the negative controls.
Negative controls should elicit no reaction or only reactions of small diameter (wheal less than 3 millimeters, erythema less than 5 millimeters).
If either the positive or negative control response does not meet the above criteria, results for allergenic extracts tested at the same time should be considered invalid and be repeated.
Intradermal Skin Testing
Preparation and Dose
For intradermal testing, use 1:20 weight/volume of Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract stock concentrate solution. Dilute the stock concentrate solution with sterile diluent [see Dosage and Administration (2.1)]. Use normal or buffered saline or normal saline with human serum albumin (HSA) diluent. If the result from the initial test dose is negative, subsequent intradermal tests using increasingly stronger doses may be performed up to the maximum recommended strength of 1:25 volume to volume dilution of the extract concentrate solution.
Inject 0.02 milliliters of the following solutions intradermally as shown in Figure 2:
Figure 2: Algorithm for Dilution of Stock Concentrate Solution of Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract for Intradermal Skin Testing
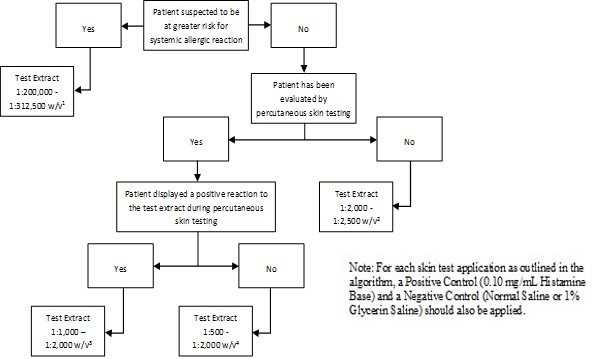
1 Corresponds to 1:10,000 - 1:15,625 volume to volume dilution of extract concentrate solution
2 Corresponds to 1:100 - 1:125 volume to volume dilution of extract concentrate solution
3 Corresponds to 1:50 - 1:100 volume to volume dilution of extract concentrate solution
4 Corresponds to 1:25 - 1:100 volume to volume dilution of extract concentrate solution
2.3 Immunotherapy
For subcutaneous administration only.
Preparation and Dose
Stock concentrates of Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract are available at 1:20 weight/volume in 50% glycerin saline for immunotherapy. Stock concentrates are diluted in normal saline, buffered saline, HSA saline, or 10% glycerin saline, depending on the patient’s reactivity to the diluent. See Table 1 and Table 2 for dilution preparation.
Administration of Immunotherapy
Administer immunotherapy by subcutaneous injection in the lateral aspect of the upper arm or thigh. Avoid injection directly into any blood vessel.
The optimal interval between doses of allergenic extract varies among individuals. Injections are usually given 1 to 2 times per week until the maintenance dose is reached, at which time the injection interval is increased to 2, then 3, and finally 4 weeks. Dosages vary by mode of administration, and by clinical response and tolerance. The minimum course of treatment may be three to five years, depending on the clinical response.
Guidelines for Immunotherapy
The initial dose of the extract should be based on the skin test reactivity. In patients suspected to be at greater risk for systemic allergic reaction by history and skin test, the initial dose of the extract should be 0.05 milliliter of a 1:20,000,000 to 1:2,000,000 weight/volume extract dilution. Patients not suspected to be at greater risk for systemic allergic reaction may be started at a 0.1 milliliter of a 1:200,000 to 1:20,000 weight/volume extract dilution.
The dose of allergenic extract is increased at each injection by no more than 50% of the previous dose, and the next increment is governed by the response to the last injection.
Select the maximum tolerated maintenance dose based on the patient's clinical response and tolerance. Doses larger than 0.2 milliliter of the stock concentrate are rarely administered because an extract mixed in 50% glycerin diluent can cause discomfort upon injection.
Dosage Modification Guidelines for Immunotherapy
The following conditions may indicate a need to withhold or reduce the dosage of immunotherapy.
- Symptoms of rhinitis and/or asthma
- Infection accompanied by fever
- Exposure to excessive amounts of clinically relevant environmental allergen prior to a scheduled injection
- Large local reactions that persist for longer than 24 hours can be an indication for repeating the previous dose or reducing the dose at the next administration
Any evidence of a systemic adverse reaction is an indication for a significant reduction (at least 75%) in the subsequent dose. Repeated systemic adverse reactions are sufficient reason for the cessation of further attempts to increase the dose.
Local adverse reactions require a decrease in the next dose by at least 50%. Proceed cautiously in subsequent dosing. In situations prompting dose reduction, once the reduced dose is tolerated, a cautious increase in dosage can be attempted.
Changing extract to a different lot or from a different manufacturer: When switching patients to a different lot of extract, or from another manufacturer’s extract, decrease the starting dose. Because manufacturing processes and sources of raw materials differ among manufacturers, the interchangeability of extracts from different manufacturers cannot be assured. In general, a dose reduction of 50 to 75% of the previous dose should be adequate, but each situation must be evaluated separately, considering the patient’s history of sensitivity, tolerance of previous injections, and other factors. Dose intervals should not exceed one week when rebuilding the dose.
Unscheduled gaps between treatments: Patients can lose tolerance for allergen injections during prolonged periods between doses, thus increasing their risk for an adverse reaction. The duration of tolerance between injections varies from patient to patient.
During the build-up phase, when patients receive injections 1 to 2 times per week, repeat or reduce the extract dosage if there has been a substantial time interval between injections. This depends on: 1) the concentration of allergen immunotherapy extract that is to be administered; 2) a previous history of systemic reactions; and 3) the degree of variation from the prescribed interval of time, with longer intervals since the last injection leading to greater reductions in the dose to be administered.
This suggested approach to dose modification, due to unscheduled gaps between treatments during the build-up phase, is not based on published evidence. The individual physician should use this or a similar protocol for the specific clinical setting.
Similarly, if unscheduled gaps occur during maintenance therapy, it may be necessary to reduce the dosage and bring the patient up to maintenance dosing using an established build-up protocol.
Changing from non-stabilized to human serum albumin (HSA) stabilized diluents: Allergenic extracts prepared with diluents containing HSA and 0.4% phenol are more stable than those prepared with diluents that do not contain stabilizers. When switching from a non-stabilized to an HSA‑stabilized diluent, consider lowering the dose for immunotherapy.
-
3 DOSAGE FORMS AND STRENGTHS
Short Ragweed Pollen Allergenic Extract solution: stock concentrate at 1:20 weight/volume with Antigen E (Amb a 1) concentration labeled in Units/milliliter.
Short and Giant Ragweed Pollen Mix Allergenic Extract solution: stock concentrate at 1:20 weight/volume with Antigen E (Amb a 1) concentration labeled in Units/milliliter.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Systemic Adverse Reactions
Serious systemic adverse reactions have occurred following the administration of other allergenic extracts and may occur in individuals following the administration of Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract in the following situations:
- Extreme sensitivity to ragweed pollen allergenic extracts
- Receipt of an accelerated immunotherapy build-up schedule (e.g., “rush” immunotherapy)
- Receipt of high doses of ragweed pollen allergenic extracts or concomitant exposure to similar environmental allergens
- Change from one allergenic lot to another allergenic lot
High-risk patients have had fatal reactions. Consider using more dilute preparations in patients suspected to be at greater risk of systemic allergic reaction [see Dosage and Administration (2.1)].
Administer these products in a healthcare setting under the supervision of a physician prepared to manage a severe systemic or a severe local allergic reaction. Observe patients in the office for at least 30 minutes following administration. 1
5.2 Epinephrine
Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract may not be suitable for patients with certain medical conditions that may reduce the ability to survive a serious allergic reaction or increase the risk of adverse reactions after epinephrine administration. Examples of these medical conditions include but are not limited to: markedly compromised lung function (either chronic or acute), unstable angina, recent myocardial infarction, significant arrhythmia, and uncontrolled hypertension.
These products may not be suitable for patients who are taking medications that can potentiate or inhibit the effect of epinephrine. These medications include:
Βeta-adrenergic blockers: Patients taking beta-adrenergic blockers may be unresponsive to the usual doses of epinephrine used to treat serious systemic reactions, including anaphylaxis. Specifically, beta-adrenergic blockers antagonize the cardiostimulating and bronchodilating effects of epinephrine.
Alpha-adrenergic blockers, ergot alkaloids: Patients taking alpha-adrenergic blockers may be unresponsive to the usual doses of epinephrine used to treat serious systemic reactions, including anaphylaxis. Specifically, alpha-adrenergic blockers antagonize the vasoconstricting and hypertensive effects of epinephrine. Similarly, ergot alkaloids may reverse the pressor effects of epinephrine.
Tricyclic antidepressants, levothyroxine sodium, monoamine oxidase inhibitors, and certain antihistamines: The adverse effects of epinephrine may be potentiated in patients taking tricyclic antidepressants, levothyroxine sodium, monoamine oxidase inhibitors, and the antihistamines chlorpheniramine, and diphenhydramine.
Cardiac glycosides, diuretics: Patients who receive epinephrine while taking cardiac glycosides or diuretics should be observed carefully for the development of cardiac arrhythmias.
5.3 Cross-Reactions and Dose Sensitivity
Since many ragweed species tend to cross-react, the total allergen content should be considered in determining the maximum maintenance dose of the mixture. The total allergen content in Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract can be comparable to that of Short Ragweed Pollen Allergenic Extract at the same weight/volume strength.
Determine the initial dilution of allergenic extract, starting dose, and progression of dosage based on the patient’s history and results of skin tests [see Dosage and Administration (2.2)]. 2 Strongly positive skin tests can be indicators for potential systemic adverse reactions.
-
6 ADVERSE REACTIONS
The most common adverse reactions, occurring in 26 to 82% of all patients who receive subcutaneous immunotherapy, are local adverse reactions at the injection site (e.g., erythema, itching, swelling, tenderness, pain). 1 Systemic adverse reactions, occurring in < 7% of patients who receive subcutaneous immunotherapy, 3,4 include generalized skin erythema, urticaria, pruritus, angioedema, rhinitis, wheezing, laryngeal edema, and hypotension. These can be fatal. 1
-
7 DRUG INTERACTIONS
7.1 Antihistamines
Do not perform skin testing with allergenic extracts within 3 to 10 days of use of first-generation H 1-histamine receptor blockers (e.g., clemastine, diphenhydramine) and second-generation antihistamines (e.g., loratadine, cetirizine). These products suppress histamine skin test reactions and could mask a positive response. 2
7.2 Topical Corticosteroids and Topical Anesthetics
Topical corticosteroids can suppress skin reactivity; therefore, discontinue use at the skin test site for 2 to 3 weeks before skin testing. Avoid use of topical local anesthetics at skin test sites as they can suppress flare responses. 2
7.3 Tricyclic Antidepressants
Tricyclic antidepressants can have potent antihistamine effects that can affect skin testing. If tricyclic medication has been recently discontinued allow 7 to 14 days before initiating skin testing. 2
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. There are no human or animal data to establish the presence or absence of Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract Allergenic Extract-associated risks during pregnancy.
8.2 Lactation
Risk Summary
It is not known whether these productsError! Document Variable not defined. are present in human milk. Data are not available to assess the effects of these extracts on the breastfed child or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for these products and any potential adverse effects on the breastfed child from the extracts or from the underlying maternal condition.
8.4 Pediatric Use
For use of these products in children younger than 5 years of age, consideration should be given to the patients’ ability to comply and cooperate with allergen immunotherapy and the potential for difficulty in communicating with the child regarding systemic reactions. 1
8.5 Geriatric Use
Data are not available to determine if subjects 65 years of age and older respond differently than younger subjects.
-
11 DESCRIPTION
Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract are sterile solutions of extracted plant pollens. Each vial contains sterile ragweed extract (short ragweed pollen or short ragweed and giant ragweed pollen mixture) at 1:20 weight/volume, 50% glycerin volume/volume, and 0.2% phenol volume/volume (preservative). Inactive ingredients include 0.25% sodium chloride for isotonicity and 0.25% sodium bicarbonate as a buffer.
These products should be clear light yellow to brownish solutions that are free of particulate matter.
These products are standardized by comparison to reference standards supplied by the Center for Biologics Evaluation and Research (CBER) of the FDA, labeled in Antigen E (Amb a 1) Units/milliliter. 5,6,7,8 The Antigen E concentration of the 1:20 weight/volume extracts is determined by radial immunodiffusion using FDA short ragweed reference standards. The extracts are labeled in both Antigen E (AgE) Units/milliliter and weight/volume extraction ratio.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The skin test reaction results from interaction of the introduced allergen and allergen-specific IgE antibodies bound to mast cells, leading to mast cell degranulation and release of histamine, tryptase and other mediators, which results in the formation of the wheal and flare.
The precise mechanisms of action of allergen immunotherapy are not known. Immunologic responses to immunotherapy include changes in allergen-specific IgE levels, allergen-specific IgG levels, and regulatory T cell responses. 1
- 14 CLINICAL STUDIES
-
15 REFERENCES
- Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011; 127(1):S1-S55.
- Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008; 100:S1-S148.
- Greenberg MA, Kaufman CR, Gonzalez GE, et al. Late and immediate systemic-allergic reactions to inhalant allergen immunotherapy. J Allergy Clin Immunol. 1986;77:865-870.
- Ragusa VF, Massolo A. Non-fatal systemic reactions to subcutaneous immunotherapy: a 20‑year experience comparison of two 10-year periods. Allerg Immunol (Paris) 2004;36:52‑55.
- Turkeltaub PC, Rastogi SC, Baer H, et al. A standardized quantitative skin-test assay of allergen potency and stability: studies on the allergen dose-response curve and effect of wheal, erythema, and patient selection on assay results. J Allergy Clin Immunol. 1982; 70(5):343-352.
- ELISA competition assay. Methods of the Allergenic Products Testing Laboratory. Rockville, MD: Laboratory of Immunobiochemistry, Division of Allergenic Products and Parasitology, Center for Biologics Evaluation and Research, Food and Drug Administration; October 1993. (On file)
- Turkeltaub PC, Rastogi SC. Quantitative intradermal procedure for evaluation of subject sensitivity to standardized allergenic extracts and for assignment of bioequivalent allergy units to reference preparations using the ID50EAL method. Rockville, MD: Laboratory of Immunobiochemistry, Division of Allergenic Products and Parasitology, Center for Biologics Evaluation and Research, Food and Drug Administration; November 1994. (On file)
- Turkeltaub PC. Assignment of bioequivalent allergy units based on biological standardization methods. Arbeiten aus dem Paul Ehrlich Institut (Bundesamt fur Sera und Impfstoffe), Band 52, Gustav Fischer Verlag, Stuttgart-New York, 1988; (82):19‑40.
- Norman PS, Winkenwerder WL, Lichtenstein LM. Immunotherapy of hay fever with ragweed antigen E: comparisons with whole pollen extract and placebos. J Allergy. 1968; 42(2):93-108.
- Arbesman CE, et al. Treatment of ragweed-sensitive patients with Antigen E and ragweed extract: Clinical and immunologic studies. J Allergy. 1967; 39(2):124.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract are supplied as stock concentrate at 1:20 weight/volume in 50% glycerin for use in percutaneous skin testing, intradermal testing, and subcutaneous immunotherapy. These products are labeled in AgE Units/milliliter and are provided in 5, 10, and 50 milliliter vials.
These products are supplied as follows:
Short Ragweed Pollen Allergenic Extract NDC Number Strength/Containier 22840-0300-5 1:20 w/v, 5 mL dropper vial for prick (skin) testing 22840-0300-2 1:20 w/v, 10 mL multi-dose vial 22840-0300-4 1:20 w/v, 50 mL multi-dose vial Short and Giant Ragweed Pollen Mix Allergenic Extract NDC Number Strength/Container 22840-0301-5 1:20 w/v, 5 mL dropper vial for prick (skin) testing 22840-0301-2 1:20 w/v, 10 mL multi-dose vial 22840-0301-4 1:20 w/v, 50 mL multi-dose vial 16.2 Storage and Handling
Maintain at 2 to 8°C (36 to 46°F) during storage and use.
Dilutions of concentrated extract result in a glycerin content of less than 50%, which can result in reduced stability. Extract dilutions at 1:100 v/v of the 1:20 weight/volume concentrate should be kept no longer than a month, and more dilute solutions no more than a week. The potency of a dilution can be checked by skin test comparison to a fresh dilution of the extract on a known ragweed-allergic patient.
-
17 PATIENT COUNSELING INFORMATION
Instruct patient to remain under observation in the office for 30 minutes or longer after an injection.
Caution patient that reactions can occur more than 30 minutes after skin testing or an injection.
Instruct patient to recognize the following symptoms as adverse reactions and to immediately return to the office or immediately seek other medical attention if any of these symptoms occur following skin testing or an injection:
- Unusual swelling and/or tenderness at the injection site
- Hives or itching of the skin
- Swelling of face and/or mouth
- Sneezing, coughing or wheezing
- Shortness of breath
- Nausea
- Dizziness or faintness
Manufacturer:
U.S. License No. 308
Greer Laboratories, Inc.
Lenoir, NC 28645 U.S.A - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SHORT AND GIANT RAGWEED POLLEN MIX
ambrosia artemisiifolia and ambrosia trifida solutionProduct Information Product Type NON-STANDARDIZED ALLERGENIC Item Code (Source) NDC:22840-0301 Route of Administration PERCUTANEOUS, SUBCUTANEOUS, INTRADERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMBROSIA ARTEMISIIFOLIA POLLEN (UNII: K20Y81ACO3) (AMBROSIA ARTEMISIIFOLIA POLLEN - UNII:K20Y81ACO3) AMBROSIA ARTEMISIIFOLIA POLLEN 0.025 g in 1 mL AMBROSIA TRIFIDA POLLEN (UNII: KU1V1898XX) (AMBROSIA TRIFIDA POLLEN - UNII:KU1V1898XX) AMBROSIA TRIFIDA POLLEN 0.025 g in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) PHENOL (UNII: 339NCG44TV) SODIUM CHLORIDE (UNII: 451W47IQ8X) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:22840-0301-5 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:22840-0301-2 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 3 NDC:22840-0301-4 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101844 09/15/1981 SHORT RAGWEED POLLEN
ambrosia artemisiifolia solutionProduct Information Product Type NON-STANDARDIZED ALLERGENIC Item Code (Source) NDC:22840-0300 Route of Administration PERCUTANEOUS, SUBCUTANEOUS, INTRADERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMBROSIA ARTEMISIIFOLIA POLLEN (UNII: K20Y81ACO3) (AMBROSIA ARTEMISIIFOLIA POLLEN - UNII:K20Y81ACO3) AMBROSIA ARTEMISIIFOLIA POLLEN 0.05 g in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) PHENOL (UNII: 339NCG44TV) GLYCERIN (UNII: PDC6A3C0OX) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:22840-0300-5 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:22840-0300-2 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 3 NDC:22840-0300-4 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101844 09/15/1981 Labeler - Greer Laboratories, Inc. (024671414) Registrant - Greer Laboroatories, Inc. (024671414) Establishment Name Address ID/FEI Business Operations Greer Laboratories, Inc. 024671414 manufacture(22840-0300)




