ASTERO- lidocaine gel
Lake Erie Medical DBA Quality Care Products LLC
Reference Label Set Id: c70f6b64-9bcd-4ce1-9df9-610285921698
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ASTERO (Lidocaine HCl USP) 4%
DESCRIPTION
A soothing hydrogel wound dressing that promotes a moist wound environment that is ideal for the healing process
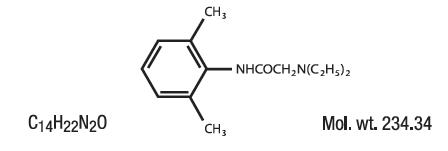
ASTERO contains Lidocaine HCl USP 4%, which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, and has the following structural formula:
INDICATIONS AND USAGE
Astero™ is a medicated Hydrogel wound dressing in a Metered Dose (M-DOSE™) bottle containing lidocaine 4%, an amide type local anesthetic, indicated for:
Painful wounds such as post-surgicalincisions, cuts and abrasions.
CONTRAINDICATIONS
Traumatized mucosa, secondary bacterial infection of the area of proposed application and known hypersensitivity to any of the components.
Lidocaine Hydrochloride USP is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type.
WARNINGS
Do not use this product if you are allergic to any ingredients. If condition worsens or does not improve within 7 days, consult a physician. Do not use on children under 2 years of age without consulting a physician.
Avoid contact with eyes. Do not use in large quantities.
For external use only.Avoid Contact with Eyes.
Keep out of reach of children.
PRECAUTIONS
If irritation or sensitivity occurs or infection appears, discontinue use and institute appropriate therapy. Astero should be used with caution in ill, elderly, debilitated patients and children who may be more sensitive to the systemic effects of Lidocaine Hydrochloride USP.
CARCINOGENESIS, MUTAGENESIS, AND IMPAIRMENT OF FERTILITY
Studies of Lidocaine Hydrochloride USP in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
USE IN PREGNANCY
Teratogenic Effects:
Teratogenic Effects of Lidocaine Hydrochloride USP. Pregnancy Category B. Reproduction studies have been performed in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by Lidocaine Hydrochloride USP. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering Lidocaine Hydrochloride USP to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
PEDIATRIC USE:
Dosage in children should be reduced, commensurate with age, body weight and physical condition. Caution must be taken to avoid over dosage when applying Astero to large areas of injured or abraded skin, since the systemic absorption of Lidocaine Hydrochloride USP may be increased under such conditions. Do not use on children under 2 unless directed by a physician.
ADVERSE REACTIONS:
Adverse experiences following the administration of Lidocaine Hydrochloride USP are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage or rapid absorption, or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient.
Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
Central Nervous System:
CNS manifestations of Lidocaine toxicity are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest. Drowsiness following the administration of Lidocaine Hydrochloride USP is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular system
Cardiovascular manifestations of Lidocaine toxicity are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions may occur as a result of sensitivity either to the local anesthetic agent or to other components in the formulation. Allergic reactions as a result of sensitivity to Lidocaine Hydrochloride USP are extremely rare and, if they occur, should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
STORAGE AND HANDLING
Store at 25ºC (77ºF); excursions permitted to 15º-30ºC (59º-86º F). See USP Controlled Room Temperature. Protect from freezing.
| ASTERO
lidocaine gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Lake Erie Medical DBA Quality Care Products LLC (831276758) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lake Erie Medical DBA Quality Care Products LLC | 831276758 | relabel(55700-421) | |
