SANATOS MULTI SYMPTOM DAYTIME- acetaminophen, dextromethorphan hbr, phenylephrine hci liquid
Pharmadel LLC
----------
Sanatos MS Daytime 6 (Deactivated)
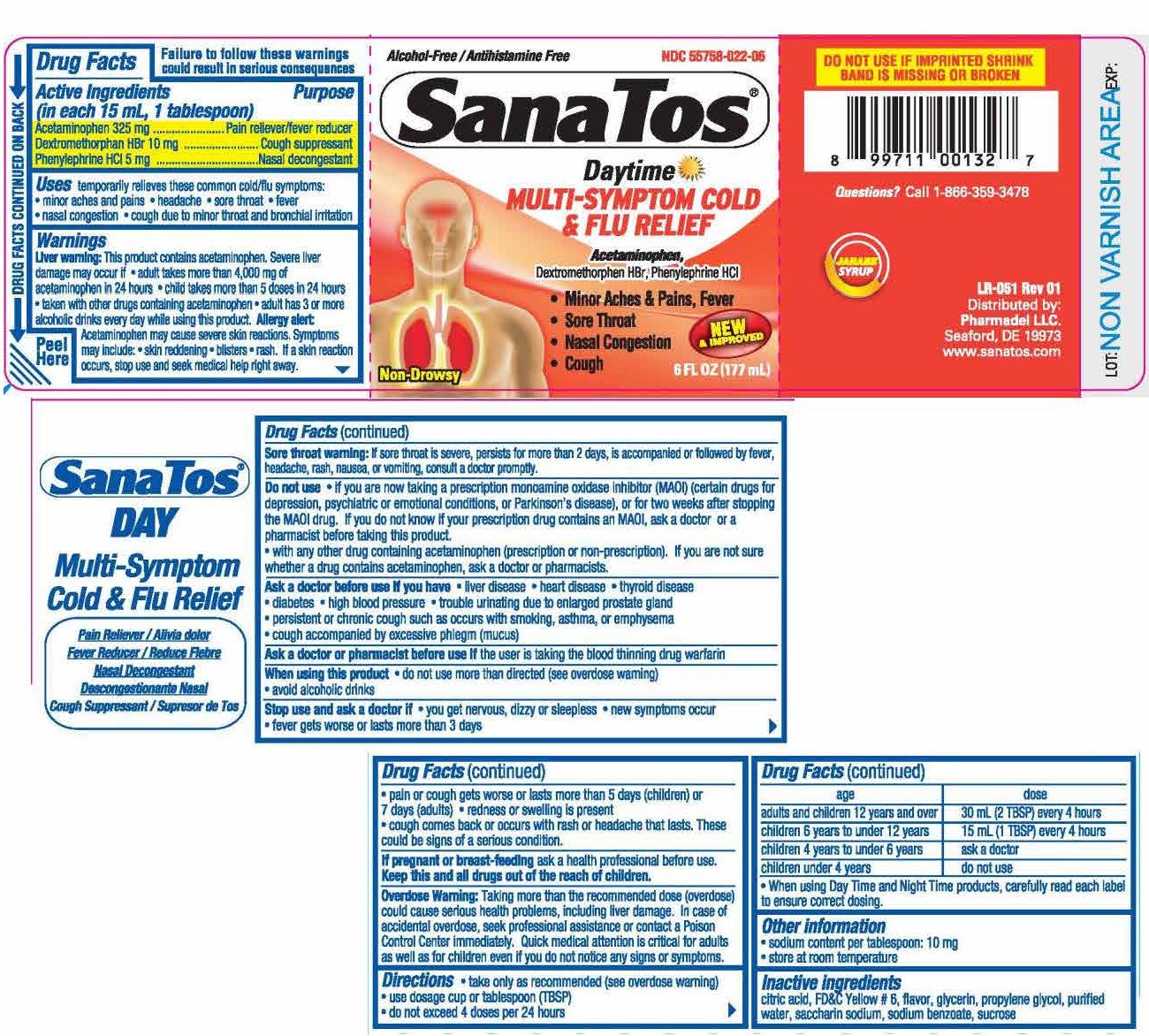
Uses
temporarily relieves common cold/flu symptoms:
- minor aches & pains
- headache
- sore throat
- fever
- nasal congestion
- cough due to minor throat and bronchial irritation
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- adults take more than 4,000 mg if acetaminophen in 24 hours
- child take more than 5 doses in 24 hours
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- Skin reddening
- Blisters
- Rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
- liver disease
- heart disease
- thyroid disease
- diabetes
- high blood pressure
- trouble urinating due to enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
- you get nervos, dizzy or sleepless
- new symptoms occur
- fever gets worse or lasts more than 3 days
- pain or cough gets worse or lasts more than 5 days (children) or 7 days (adults)
- redness or swelling is present
- cough comes back or occurs with rash or headache that lasts
These could be signs of a serious condition.
Directions
- take only as recommended (see overdose warning)
- use dosage cup or tablespoon (TBSP)
- do not exceed 4 doses per 24 hrs
| age | dose |
| adults & children 12 years & over | 30 mL (2 TBSP) every 6 hours |
| children 6 years to under 12 years | 15 mL (1 TTBSP) every 4 hours |
| children 4 years to under 6 years | ask a doctor |
| children under 4 years | do not use |
- when using daytime and nighttime products, carefully read each label to ensure correct dosing.
Other information
- sodium content per tablespoon: 10 mg
- store at room temperature
TAMPER EVIDENT: Do not use if imprinted band is missing or broken.
| SANATOS MULTI SYMPTOM DAYTIME
acetaminophen, dextromethorphan hbr, phenylephrine hci liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmadel LLC (030129680) |
Revised: 11/2023
Document Id: 0ab262c2-49cf-22da-e063-6394a90a4650
Set id: 60506714-179c-6714-e053-2991aa0a51b3
Version: 5
Effective Time: 20231121
Pharmadel LLC