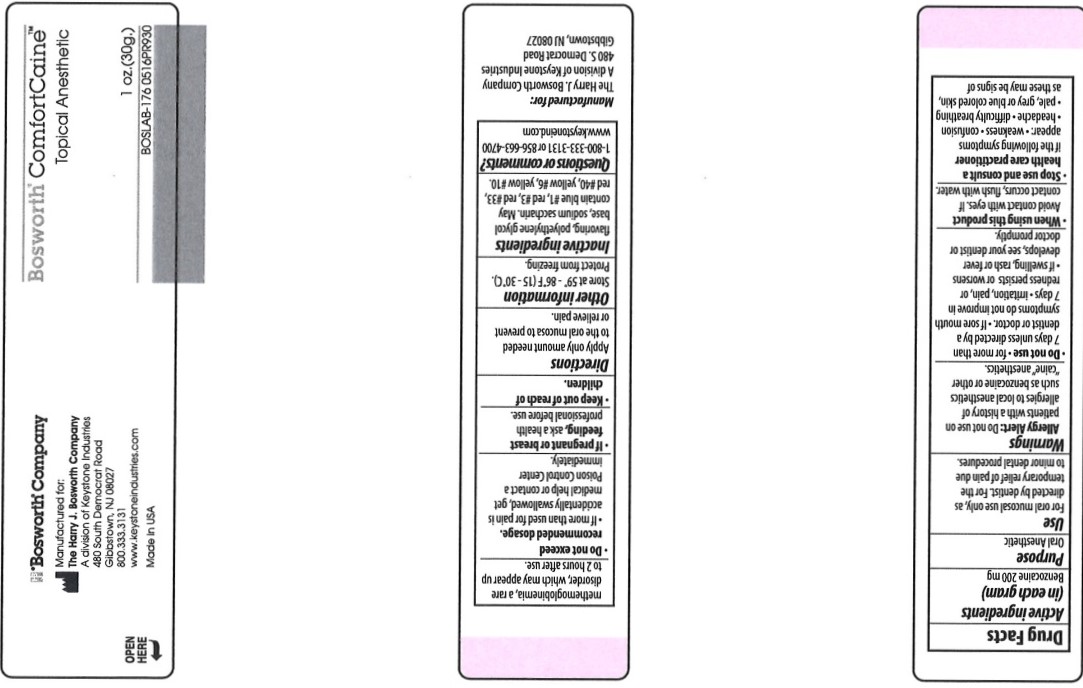
COMFORTCAINE- benzocaine gel
Mycone Dental Supply Co., Inc DBA Keystone Industries and Deepack Products Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Comfortcaine - Strawberry
Uses
For oral mucosal use only, as directed by dentist. For the temporary relief of pain due to minor dental procedures.
Warnings
Allergy Alert: Do not use on patients with a history of allergies to local anesthetics such as benzocaine or other "caine" anesthetics.
Do not use
- for more than 7 days unless directed by a dentist or doctor.
- If sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- if swelling, rash or fever develops, see your dentist or doctor promptly.
Stop use and consult a health care pracitioner
if the following symptoms appear.
- weakness
- confusion
- headache
- difficulty breathing
- pale
- grey or blue colored skin, as this may be signs of methemoglobinemia, a rare disorder, which may appear up to 2 hours after use.
Do not exceed recommended dosage.
- If more than used for pain is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
| COMFORTCAINE
benzocaine gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Mycone Dental Supply Co., Inc DBA Keystone Industries and Deepack Products Inc. (014769301) |
Revised: 5/2018
Document Id: 6cf42857-63fc-a60b-e053-2991aa0aa2f1
Set id: 603ef657-b34e-633f-e053-2a91aa0a847f
Version: 2
Effective Time: 20180524
Mycone Dental Supply Co., Inc DBA Keystone Industries and Deepack Products Inc.