AMMONIA N 13- ammonia n-13 injection
The University of Texas MD Anderson Cancer Center
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Ammonia N 13 Injection safely and effectively. See full prescribing information for Ammonia N 13 Injection. Ammonia N 13 Injection for intravenous use. Initial U.S. Approval: 2007 INDICATIONS AND USAGE(1)
These highlights do not include all the information needed to use Ammonia N 13 Injection safely and effectively. See full prescribing information for Ammonia N 13 Injection. Ammonia N 13 Injection for intravenous use.
(1) Ammonia N 13 Injection is a radioactive diagnostic agent for Positron Emission Tomography (PET) indicated for diagnostic PET imaging of the myocardium under rest or pharmacologic stress conditions to evaluate myocardial perfusion in patients with suspected or existing coronary artery disease (1). (1) DOSAGE AND ADMINISTRATIONRest Imaging Study (2.1): (2) Aseptically withdraw Ammonia N 13 Injection from its container and administer 10-20 mCi (0.368 – 0.736 GBq) as a bolus through a catheter inserted into a large peripheral vein. (2) Start imaging 3 minutes after the injection and acquire images for a total of 10-20 minutes. (2) Stress Imaging Study (2.2): (2) If a rest imaging study is performed, begin the stress imaging study 40 minutes or more after the first Ammonia N13 injection to allow sufficient isotope decay.
Patient Preparation (2.3): (2) To increase renal clearance of radioactivity and to minimize radiation dose to the bladder, hydrate the patient before the procedure and encourage voiding as soon as each image acquisition is completed and as often as possible thereafter for at least one hour. (2) DOSAGE FORMS AND STRENGTHSGlass vial containing 0.138-1.387 GBq (3.75-37.5 mCi/mL) of Ammonia N 13 Injection in aqueous 0.9 % sodium chloride solution (approximately 4 mL volume) (3). (3) CONTRAINDICATIONSNone (4) (4) WARNINGS AND PRECAUTIONSAmmonia N 13 Injection may increase the risk of cancer. Use the smallest dose necessary for imaging and ensure safe handling to protect the patient and health care worker (5). (5) ADVERSE REACTIONSNo adverse reactions have been reported for Ammonia N 13 Injection based on a review of the published literature, publicly available reference sources, and adverse drug reaction reporting system(6). (6) To report SUSPECTED ADVERSE REACTIONS, contact The University of Texas MD Anderson Cancer Center, Cyclotron Radiochemistry Facility at 1-713-563-5455 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6) USE IN SPECIFIC POPULATIONS
(8) See 17 for PATIENT COUNSELING INFORMATION (8) See 17 for PATIENT COUNSELING INFORMATION. Revised: 12/2017 |
FULL PRESCRIBING INFORMATION
1 INDICATION AND USAGE
These highlights do not include all the information needed to use Ammonia N 13 Injection safely and effectively. See full prescribing information for Ammonia N 13 Injection. Ammonia N 13 Injection for intravenous use.
Initial U.S. Approval: 2007
Ammonia N 13 Injection is indicated for diagnostic Positron Emission Tomography (PET) imaging of the myocardium under rest or pharmacologic stress conditions to evaluate myocardial perfusion in patients with suspected or existing coronary artery disease.
2 DOSAGE FORMS AND ADMINISTRATION
2.1 Rest Imaging Study
- Aseptically withdraw Ammonia N 13 Injection from its container and administer 10-20 mCi (0.368 – 0.736 GBq) as a bolus through a catheter inserted into a large peripheral vein.
2.2 Stress Imaging Study
- If a rest imaging study is performed, begin the stress imaging study 40 minutes or more after the first Ammonia N 13 injection to allow sufficient isotope decay.
- Administer a pharmacologic stress-inducing drug in accordance with its labeling.
- Aseptically withdraw Ammonia N 13 Injection from its container and administer 10-20 mCi (0.368 – 0.736 GBq) of Ammonia N 13 Injection as a bolus at 8 minutes after the administration of the pharmacologic stress-inducing drug.
- Start imaging 3 minutes after the Ammonia N 13 Injection and acquire images for a total of 10-20 minutes.
2.3 Patient Preparation
To increase renal clearance of radioactivity and to minimize radiation dose to the bladder, ensure that the patient is well hydrated before the procedure and encourage voiding as soon as a study is completed and as often as possible thereafter for at least one hour.
2.4 Radiation Dosimetry
The converted radiation absorbed doses in rem/mCi are shown in Table 1. These estimates are calculated from the Task Group of
Committee 2 of the International Commission on Radiation Protection.
1
Table 1: N 13 Absorbed Radiation Dose Per Unit Activity (rem/mCi) for Adults and Pediatric Groups
| Organ | Age (years) | Age (years) | Age (years) | Age (years) | Age (years) |
| Adult | 15 | 10 | 5 | 1 | |
| Adrenals | 0.085 | 0.0096 | 0.016 | 0.025 | 0.048 |
| Bladder wall | 0.030 | 0.037 | 0.056 | 0.089 | 0.17 |
| Bone surfaces | 0.0059 | 0.0070 | 0.011 | 0.019 | 0.037 |
| Brain | 0.016 | 0.016 | 0.017 | 0.019 | 0.027 |
| Breast | 0.0067 | 0.0067 | 0.010 | 0.017 | 0.033 |
| Stomach wall | 0.0063 | 0.0078 | 0.012 | 0.019 | 0.037 |
| Small intenstine | 0.0067 | 0.0081 | 0.013 | 0.021 | 0.041 |
| *ULI | 0.0067 | 0.0078 | 0.013 | 0.021 | 0.037 |
| **LLI | 0.0070 | 0.0078 | 0.013 | 0.020 | 0.037 |
| Heart | 0.0078 | 0.0096 | 0.015 | 0.023 | 0.041 |
| Kidneys | 0.017 | 0.021 | 0.031 | 0.048 | 0.089 |
| Liver | 0.015 | 0.018 | 0.029 | 0.044 | 0.085 |
| Lungs | 0.0093 | 0.011 | 0.018 | 0.029 | 0.056 |
| Ovaries | 0.0063 | 0.0085 | 0.014 | 0.021 | 0.041 |
| Pancreas | 0.0070 | 0.0085 | 0.014 | 0.021 | 0.041 |
| Red marrow | 0.0063 | 0.0078 | 0.012 | 0.020 | 0.037 |
| Spleen | 0.0093 | 0.011 | 0.019 | 0.030 | 0.056 |
| Testes | 0.0067 | 0.0070 | 0.011 | 0.018 | 0.035 |
| Thyroid | 0.0063 | 0.0081 | 0.013 | 0.021 | 0.041 |
| Uterus | 0.0070 | 0.0089 | 0.014 | 0.023 | 0.041 |
| Other tissues | 0.0059 | 0.0070 | 0.011 | 0.018 | 0.035 |
*Upper large intestine, **Lower large intestine
2.5 Drug Handling
- Inspect Ammonia N 13 Injection visually for particulate matter and discoloration before administration, whenever solution and container permit.
- Do not administer Ammonia N 13 Injection containing particulate matter or discoloration; dispose of these unacceptable or unused preparations in a safe manner, in compliance with applicable regulations.
- Wear waterproof gloves and effective shielding when handling Ammonia N 13 Injection.
- Use aseptic technique to maintain sterility during all operations involved in the manipulation and administration of Ammonia N 13 Injection. The contents of each vial are sterile and non-pyrogenic.
- Use appropriate safety measures, including shielding, consistent with proper patient management to avoid unnecessary radiation exposure to the patient, occupational workers, clinical personnel, and other persons.
- Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
- Before administration of Ammonia N 13 Injection, assay the dose in a properly calibrated dose calibrator.
3 DOSAGE FORMS AND STRENGTHS
Glass vial (10 mL) containing 0.138-1.387 GBq (3.75-37.5 mCi/mL) of Ammonia N 13 Injection in aqueous 0.9 % sodium chloride solution (approximately 4 mL volume) that is suitable for intravenous administration.
6 ADVERSE REACTIONS
No adverse reactions have been reported for Ammonia N 13 Injection based on a review of the published literature, publicly available reference sources, and adverse drug reaction reporting systems. However, the completeness of these sources is not known.
7 DRUG INTERACTIONS
The possibility of interactions of Ammonia N 13 Injection with other drugs taken by patients undergoing PET imaging has not been studied.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with Ammonia N 13 Injection. It is also not known whether Ammonia N 13 Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Ammonia N 13 Injection should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for radiation exposure to nursing infants from Ammonia N 13 Injection, use alternative infant nutrition sources (e.g. stored breast milk or infant formula) for 2 hours (>10 half-lives of radioactive decay for N 13 isotope) after administration of the drug or avoid use of the drug, taking into account the importance of the drug to the mother.
11 DESCRIPTION
11.1 Chemical Characteristics
Ammonia N 13 Injection is a positron emitting radiopharmaceutical that is used for diagnostic purposes in conjunction with positron emission tomography (PET) imaging. The active ingredient, [ 13N] ammonia, has the molecular formula of 13NH 3 with a molecular weight of 16.02, and has the following chemical structure:
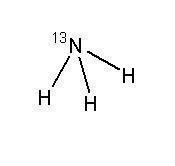
11.2 Physical Characteristics
Nitrogen N13 decays by emitting positron to Carbon C13 (stable) and has a physical half-life of 9.96 minutes. The principal photons useful for imaging are the dual 511 keV gamma photons that are produced and emitted simultaneously in opposite direction when the positron interacts with an electron (Table 2).
Table 2: Principal Radiation Emission Data for Nitrogen 13
| Radiation/Emission | % Per Disintegration | Energy |
| Positron (+) | 100 | 1190 keV (Max.) |
| Gamma (±) * | 200 | 511 keV |
*Produced by positron annihilation
The specific gamma ray constant (point source air kerma coefficient) for nitrogen N13 is 5.9 R/hr/mCi (1.39 x 10 -6 Gy/hr/kBq) at 1 cm. The half-value layer (HVL) of lead (Pb) for 511 keV photons is 4 mm. Selected coefficients of attenuation are listed in Table 3 as a function of lead shield thickness. For example, the use of 39 mm thickness of lead will attenuate the external radiation by a factor of about 1000.
Table 3: Radiation Attenuation of 511 keV Photons by lead (Pb) Shielding
| Shield Thickness (Pb) mm | Coefficient of Attenuation |
| 4 | 0.5 |
| 8 | 0.25 |
| 13 | 0.1 |
| 26 | 0.01 |
| 39 | 0.001 |
| 52 | 0.0001 |
Table 4 lists fractions remaining at selected time intervals from the calibration time. This information may be used to correct for physical decay of the radionuclide.
Table 4: Physical Decay Chart for Nitrogen N 13
| Minutes | Fraction Remaining |
| 0 * | 1.000 |
| 5 | 0.706 |
| 10 | 0.499 |
| 15 | 0.352 |
| 20 | 0.249 |
| 25 | 0.176 |
| 30 | 0.124 |
*Calibration time
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ammonia N 13 Injection is a radiolabeled analog of ammonia that is distributed to all organs of the body after intravenous administration. It is extracted from the blood in the coronary capillaries into the myocardial cells where it is metabolized to glutamine N 13 and retained in the cells. The presence of ammonia N 13 and glutamine N 13 in the myocardium allows for PET imaging of the myocardium.
12.2 Pharmacodynamics
Following intravenous injection, ammonia N 13 enters the myocardium through the coronary arteries. The PET technique measures myocardial blood flow based on the assumption of a three compartmental disposition of intravenous ammonia N 13 in the myocardium. In this model, the value of the rate constant, which represents the delivery of blood to myocardium, and the fraction of ammonia N 13 extracted into the myocardial cells, is a measure of myocardial blood flow. Optimal PET imaging of the myocardium is generally achieved between 10 to 20 minutes after administration.
12.3 Pharmacokinetics
Following intravenous injection, Ammonia N 13 Injection is cleared from the blood with a biologic half-life of about 2.84 minutes (effective half-life of about 2.21 minutes). In the myocardium, its biologic half-life has been estimated to be less than 2 minutes (effective half-life less than 1.67 minutes).
The mass dose of Ammonia N 13 Injection is very small as compared to the normal range of ammonia in the blood (0.72-3.30 mg) in a healthy adult man [see Description (11.1)].
Plasma protein binding of ammonia N 13 or its N 13 metabolites has not been studied.
Ammonia N 13 undergoes a five-enzyme step metabolism in the liver to yield urea N 13 (the main circulating metabolite). It is also metabolized to glutamine N 13 (the main metabolite in tissues) by glutamine synthesis in the skeletal muscles, liver, brain, myocardium, and other organs. Other metabolites of ammonia N 13 include small amounts of N 13 amino acid anions (acidic amino acids) in the forms of glutamate N 13 or aspartate N 13.
Ammonia N 13 is eliminated from the body by urinary excretion mainly as urea N 13.
The pharmacokinetics of Ammonia N 13 Injection have not been studied in renally impaired, hepatically impaired, or pediatric patients.
14 CLINICAL STUDIES
In a descriptive, prospective, blinded image interpretation study 2 of adult patients with known or suspected coronary artery disease, myocardial perfusion deficits in stress and rest PET images obtained with Ammonia N 13 (N=111) or Rubidium 82 (N=82) were compared to changes in stenosis flow reserve (SFR) as determined by coronary angiography. The principal outcome of the study was the evaluation of PET defect severity relative to SFR.
PET perfusion defects at rest and stress for seven cardiac regions (anterior, apical, anteroseptal, posteroseptal, anterolateral, posterolateral, and inferior walls) were graded on a 0 to 5 scale defined as normal (0), possible (1), probable (2), mild (3), moderate (4), and severe (5) defects. Coronary angiograms were used to measure absolute and relative stenosis dimensions and to calculate stenosis flow reserve defined as the maximum value of flow at maximum coronary vasodilatation relative to rest flow under standardized hemodynamic conditions. SFR scores ranged from 0 (total occlusion) to 5 (normal). With increasing impairment of flow reserve, the subjective PET defect severity increased. A PET defect score of 2 or higher was positively correlated with flow reserve impairment (SFR<3).
15 REFERENCES
- Annals of the ICRP. Publication 53. Radiation dose to patients from radiopharmaceuticals. New York: Pergamon Press, 1988.
- Demer, L.L.K.L.Gould, R.A.Goldstein, R.L.Kirkeeide, N.A.Mullani, R.W. Smalling, A.Nishikawa, and M.E.Merhige. Assessment of coronary artery disease severity by PET: Comparison with quantitative arteriography in 193 patients. Circulation 1989; 79: 825-35.
16 HOW SUPPLIED/STORAGE AND HANDLING
Ammonia N 13 Injection is packaged in 10 mL multiple dose glass vial containing between 0.555 GBq to 5.55 GBq (15 mCi to 150 mCi) of [ 13N] ammonia, at the end of synthesis (EOS) reference time, in 0.9% sodium chloride injection solution in approximately 4 mL volume. The recommended dose of radioactivity (10-20 mCi) is associated with a theoretical mass dose of 0.5 – 1.0 picomoles (8.47-16.94 picograms) of Ammonia.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).Use the solution within 60 minutes of the End of Synthesis (EOS)
calibration.
17 PATIENT COUNSELING INFORMATION
17.2 Post-study Voiding
Instruct patients to void after completion of each image acquisition session and as often as possible for one hour after the PET scan ends.
| AMMONIA
N 13
ammonia n-13 injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| Labeler - The University of Texas MD Anderson Cancer Center (016568851) |
| Registrant - The University of Texas MD Anderson Cancer Center (016568851) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The University of Texas MD Anderson Cancer Center | 016568851 | positron emission tomography drug production(60215-201) | |
