Label: TERBINAFINE HYDROCHLORIDE cream
- NDC Code(s): 68788-9814-1
- Packager: Preferred Pharmaceuticals, Inc
- This is a repackaged label.
- Source NDC Code(s): 51672-2080
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
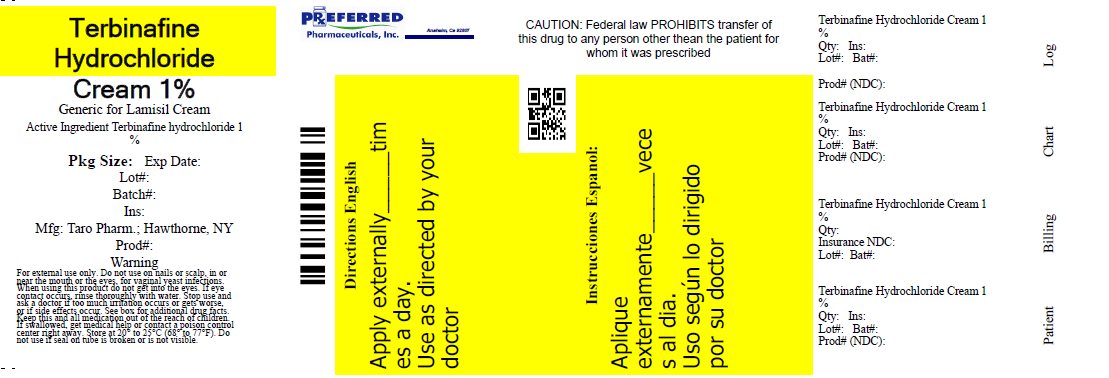
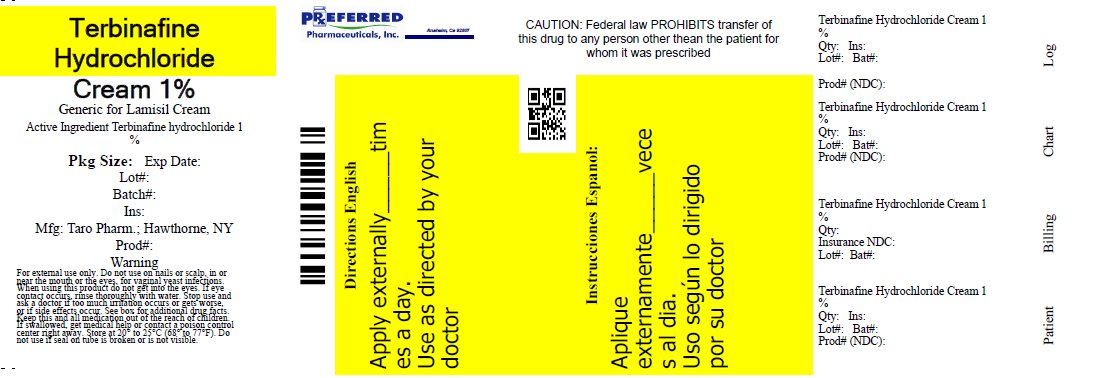
- PRINCIPAL DISPLAY PANEL - 30 g Carton
-
INGREDIENTS AND APPEARANCE
TERBINAFINE HYDROCHLORIDE
terbinafine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-9814(NDC:51672-2080) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Terbinafine Hydrochloride (UNII: 012C11ZU6G) (Terbinafine - UNII:G7RIW8S0XP) Terbinafine Hydrochloride 1 g in 100 g Inactive Ingredients Ingredient Name Strength benzyl alcohol (UNII: LKG8494WBH) cetyl alcohol (UNII: 936JST6JCN) cetyl palmitate (UNII: 5ZA2S6B08X) isopropyl myristate (UNII: 0RE8K4LNJS) polysorbate 60 (UNII: CAL22UVI4M) water (UNII: 059QF0KO0R) sodium hydroxide (UNII: 55X04QC32I) sorbitan monostearate (UNII: NVZ4I0H58X) stearyl alcohol (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-9814-1 15 g in 1 TUBE; Type 0: Not a Combination Product 04/05/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077511 04/05/2012 Labeler - Preferred Pharmaceuticals, Inc (791119022) Registrant - Preferred Pharmaceuticals, Inc (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals, Inc 791119022 RELABEL(68788-9814)