TRAMADOL HYDROCHLORIDE- tramadol hydrochloride tablet
Lake Erie Medical DBA Quality Care Products LLC
----------
TRAMADOL HYDROCHLORIDE TABLETS
50 mg - Rx only
DESCRIPTION
Tramadol hydrochloride tablet is a centrally acting analgesic. The chemical name for tramadol hydrochloride is (±)cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride. Its structural formula is:
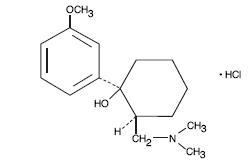
Molecular formula is C16H13NO2•HCl
The molecular weight of tramadol hydrochloride is 299.8. Tramadol hydrochloride is a white, bitter, crystalline and odorless powder. It is readily soluble in water and ethanol and has a pKa of 9.41. The n-octanol/water log partition coefficient (logP) is 1.35 at pH7. Tramadol hydrochloride tablets, for oral administration contain 50 mg of tramadol hydrochloride. In addition, each tablet contains the following inactive ingredients: pregelatinized starch, lactose anhydrous, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium starch glycolate, titanium dioxide.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Tramadol hydrochloride is a centrally acting synthetic opioid analgesic. Although its mode of action is not completely understood, from animal tests, at least two complementary mechanisms appear applicable: binding of parent and M1 metabolite to µ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin.
Opioid activity is due to both low affinity binding of the parent compound and higher affinity binding of the O-demethylated metabolite M1 to µ-opioid receptors. In animal models, M1 is up to 6 times more potent than tramadol in producing analgesia and 200 times more potent in µ-opioid binding. Tramadol-induced analgesia is only partially antagonized by the opiate antagonist naloxone in several animal tests. The relative contribution of both tramadol and M1 to human analgesia is dependent upon the plasma concentrations of each compound (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
Tramadol has been shown to inhibit reuptake of norepinephrine and serotonin in vitro, as have some other opioid analgesics. These mechanisms may contribute independently to the overall analgesic profile of tramadol hydrochloride tablets. Analgesia in humans begins approximately within one hour after administration and reaches a peak in approximately two to three hours.
Apart from analgesia, tramadol hydrochloride tablets administration may produce a constellation of symptoms (including dizziness, somnolence, nausea, constipation, sweating and pruritus) similar to that of other opioids. In contrast to morphine, tramadol has not been shown to cause histamine release. At therapeutic doses, tramadol hydrochloride tablets have no effect on heart rate, left-ventricular function or cardiac index. Orthostatic hypotension has been observed.
Pharmacokinetics
The analgesic activity of tramadol hydrochloride tablets is due to both parent drug and the M1 metabolite (see CLINICAL PHARMACOLOGY, Pharmacodynamics). Tramadol is administered as a racemate and both the [-] and [+] forms of both tramadol and M1 are detected in the circulation. Linear pharmacokinetics have been observed following multiple doses of 50 and 100 mg to steady-state.
Absorption:
The mean absolute bioavailability of a 100 mg oral dose is approximately 75%. The mean peak plasma concentration of racemic tramadol and M1 occurs at two and three hours, respectively, after administration in healthy adults. In general, both enantiomers of tramadol and M1 follow a parallel time course in the body following single and multiple doses although small differences (~10%) exist in the absolute amount of each enantiomer present.
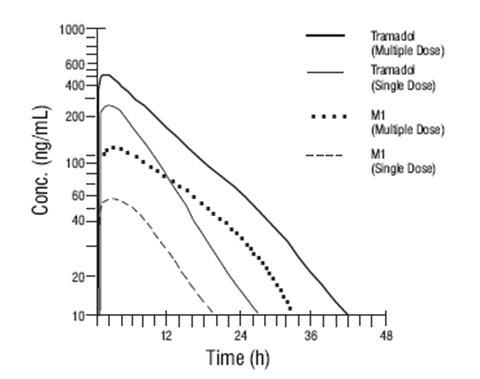
Steady-state plasma concentrations of both tramadol and M1 are achieved within two days with four times per day dosing. There is no evidence of self-induction (see Figure 1 and Table 1 below).
Figure 1: Mean Tramadol and M1 Plasma Concentration Profiles after a Single 100 mg Oral Dose and after Twenty-Nine 100 mg Oral Doses of Tramadol HCl given four times per day.
| Population/ Dosage Regimena | Parent Drug/ Metabolite | Peak Conc. (ng/mL) | Time to Peak (hrs) | Clearance/Fb
(mL/min/kg) | t 1/2 (hrs) |
|---|---|---|---|---|---|
| a SD = Single dose, MD = Multiple dose, p.o. = Oral administration, i.v. = Intravenous administration, qid = Four times daily b F represents the oral bioavailability of tramadol c Not applicable d Not measured |
|||||
| Healthy Adults, 100 mg q.i.d., MD p.o. | Tramadol | 592 (30) | 2.3 (61) | 5.90 (25) | 6.7 (15) |
| M1 | 110 (29) | 2.4 (46) | c | 7.0 (14) | |
| Healthy Adults, 100 mg SD p.o. | Tramadol | 308 (25) | 1.6 (63) | 8.50 (31) | 5.6 (20) |
| M1 | 55.0 (36) | 3.0 (51) | c | 6.7 (16) | |
| Geriatric, (> 75 yrs) 50 mg SD p.o. | Tramadol | 208 (31) | 2.1 (19) | 6.89 (25) | 7.0 (23) |
| M1 | d | d | c | d | |
| Hepatic Impaired 50 mg SD p.o. | Tramadol | 217 (11) | 1.9 (16) | 4.23 (56) | 13.3 (11) |
| M1 | 19.4 (12) | 9.8 (20) | c | 18.5 (15) | |
| Renal Impaired CLcr 10-30 mL/min 100 mg SD i.v. | Tramadol | c | c | 4.23 (54) | 10.6 (31) |
| M1 | c | c | c | 11.5 (40) | |
| Renal Impaired CLcr <5 mL/min 100 mg SD i.v. | Tramadol | c | c | 3.73 (17) | 11.0 (29) |
| M1 | c | c | c | 16.9 (18) | |
Food Effects:
Oral administration of tramadol hydrochloride tablets with food does not significantly affect its rate or extent of absorption, therefore, tramadol hydrochloride tablets can be administered without regard to food.
Distribution:
The volume of distribution of tramadol was 2.6 and 2.9 liters/kg in male and female subjects, respectively, following a 100 mg intravenous dose. The binding of tramadol to human plasma proteins is approximately 20% and binding also appears to be independent of concentration up to 10 µg/mL. Saturation of plasma protein binding occurs only at concentrations outside the clinically relevant range.
Metabolism:
Tramadol is extensively metabolized after oral administration by a number of pathways, including CYP2D6 and CYP3A4, as well as by conjugation of parent and metabolites. Approximately 30% of the dose is excreted in the urine as unchanged drug, whereas 60% of the dose is excreted as metabolites. The remainder is excreted either as unidentified or as unextractable metabolites. The major metabolic pathways appear to be N- and O-demethylation and glucuronidation or sulfation in the liver. One metabolite (O-desmethyltramadol, denoted M1) is pharmacologically active in animal models. Formation of M1 is dependent on CYP2D6 and as such is subject to inhibition, which may affect the therapeutic response (see PRECAUTIONS-Drug Interaction).
Approximately 7% of the population has reduced activity of the CYP2D6 isoenzyme of cytochrome P-450. These individuals are “poor metabolizers” of debrisoquine, dextromethorphan, tricyclic antidepressants, among other drugs. Based on a population PK analysis of Phase l studies in healthy subjects, concentrations of tramadol were approximately 20% higher in “poor metabolizers” versus “extensive metabolizers”, while M1 concentrations were 40% lower. Concomitant therapy with inhibitors of CYP2D6 such as fluoxetine, paroxetine, and quinidine could result in significant drug interactions. In vitro drug interaction studies in human liver microsomes indicate that inhibitors of CYP2D6 such as fluoxetine and its metabolite norfluoxetine, amitriptyline and quinidine inhibit the metabolism of tramadol to various degrees, suggesting that concomitant administration of these compounds could result in increases in tramadol concentrations and decreased concentrations of M1. The full pharmacological impact of these alterations in terms of either efficacy or safety is unknown. Concomitant use of SEROTONIN re-uptake INHIBITORS and MAO INHIBITORS may enhance the risk of adverse events, including seizure (see WARNINGS) and serotonin syndrome.
Elimination:
Tramadol is eliminated primarily through metabolism by the liver and the metabolites are eliminated primarily by the kidneys. The mean terminal plasma elimination half-lives of racemic tramadol and racemic M1 are 6.3 ± 1.4 and 7.4 ± 1.4 hours, respectively. The plasma elimination half-life of racemic tramadol increased from approximately six hours to seven hours upon multiple dosing.
Clinical Studies
Tramadol hydrochloride tablets have been given in single oral doses of 50, 75, and 100 mg to patients with pain following surgical procedures and pain following oral surgery (extraction of impacted molars).
In single-dose models of pain following oral surgery, pain relief was demonstrated in some patients at doses of 50 mg and 75 mg. A dose of 100 mg tramadol hydrochloride tablets tended to provide analgesia superior to codeine sulfate 60 mg, but it was not as effective as the combination of aspirin 650 mg with codeine phosphate 60 mg.
Tramadol hydrochloride tablets have been studied in three long-term controlled trials involving a total of 820 patients, with 530 patients receiving tramadol hydrochloride tablets. Patients with a variety of chronic painful conditions were studied in double-blind trials of one to three months duration. Average daily doses of approximately 250 mg of tramadol hydrochloride tablets in divided doses were generally comparable to five doses of acetaminophen 300 mg with codeine phosphate 30 mg (TYLENOL® with Codeine #3) daily, five doses of aspirin 325 mg with codeine phosphate 30 mg daily, or two to three doses of acetaminophen 500 mg with oxycodone hydrochloride 5 mg (TYLOX®) daily. [TYLENOL® is the registered trademark of McNeil Consumer Healthcare and TYLOX® is the registered trademark of RW Johnson].
Titration Trials
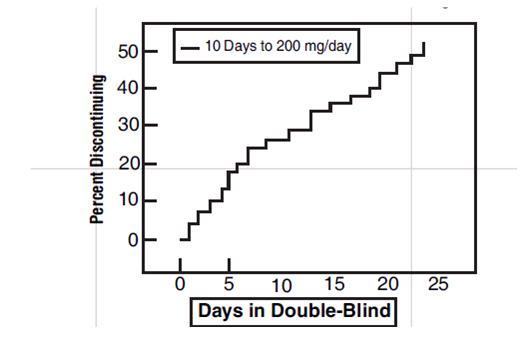
In a randomized, blinded clinical study with 129 to 132 patients per group, a 10-day titration to a daily tramadol hydrochloride dose of 200 mg (50 mg four times per day), attained in 50 mg increments every 3 days, was found to result in fewer discontinuations due to dizziness or vertigo than titration over only 4 days or no titration.
Figure 2:Protocol CAPSS-047
Time to Discontinuation Due to Nausea/Vomiting
INDICATIONS AND USAGE
Tramadol hydrochloride tablets are indicated for the management of moderate to moderately severe pain in adults.
CONTRAINDICATIONS
Tramadol hydrochloride tablets should not be administered to patients who have previously demonstrated hypersensitivity to tramadol, any other component of this product or opioids. Tramadol hydrochloride is contraindicated in any situation where opioids are contraindicated, including acute intoxication with any of the following: alcohol, hypnotics, narcotics, centrally acting analgesics, opioids or psychotropic drugs. Tramadol may worsen central nervous system and respiratory depression in these patients.
ADVERSE REACTIONS
Tramadol hydrochloride tablets were administered to 550 patients during the double-blind or open-label extension periods in U.S. studies of chronic nonmalignant pain. Of these patients, 375 were 65 years old or older. Table 2 reports the cumulative incidence rate of adverse reactions by 7, 30, and 90 days for the most frequent reactions (5% or more by 7 days). The most frequently reported events were in the central nervous system and gastrointestinal system. Although the reactions listed in the table are felt to be probably related to tramadol hydrochloride tablets administration, the reported rates also include some events that may have been due to underlying disease or concomitant medication. The overall incidence rates of adverse experiences in these trials were similar for tramadol hydrochloride tablets and the active control groups, TYLENOL® with codeine #3 (acetaminophen 300 mg with codeine phosphate 30 mg), and aspirin 325 mg with codeine phosphate 30 mg however the rates of withdrawals due to adverse events appeared to be higher in the tramadol hydrochloride groups. [TYLENOL® is the registered trademark of McNeil Consumer Healthcare and TYLOX® is the registered trademark of RW Johnson].
|
| Up to 7 Days | Up to 30 Days | Up to 90 Days |
|---|---|---|---|
|
1 “CNS Stimulation” is a composite of nervousness, anxiety, agitation, tremor, spasticity, euphoria, emotional lability and hallucinations. |
|||
| Dizziness/Vertigo | 26% | 31% | 33% |
| Nausea | 24% | 34% | 40% |
| Constipation | 24% | 38% | 46% |
| Headache | 18% | 26% | 32% |
| Somnolence | 16% | 23% | 25% |
| Vomiting | 9% | 13% | 17% |
| Pruritus | 8% | 10% | 11% |
| “CNS Stimulation” 1
| 7% | 11% | 14% |
| Asthenia | 6% | 11% | 12% |
| Sweating | 6% | 7% | 9% |
| Dyspepsia | 5% | 9% | 13% |
| Dry Mouth | 5% | 9% | 10% |
| Diarrhea | 5% | 6% | 10% |
Incidence 1% to less than 5%, possibly causally related: the following lists adverse reactions that occurred with an incidence of 1% to less than 5% in clinical trials, and for which the possibility of a causal relationship with tramadol hydrochloride tablets exists.
Body as a Whole: Malaise.
Cardiovascular: Vasodilation.
Central Nervous System: Anxiety, Confusion, Coordination disturbance, Euphoria, Miosis, Nervousness, Sleep disorder.
Gastrointestinal: Abdominal pain, Anorexia, Flatulence.
Musculoskeletal: Hypertonia.
Skin: Rash.
Special Senses: Visual disturbance.
Urogenital: Menopausal symptoms, Urinary frequency, Urinary retention.
Incidence less than 1%, possibly causally related: the following lists adverse reactions that occurred with an incidence of less than 1% in clinical trials and/or reported in post-marketing experience.
Body as a Whole: Accidental injury, Allergic reaction, Anaphylaxis, Death, Suicidal tendency, Weight loss, Serotonin syndrome (mental status change, hyperreflexia, fever, shivering, tremor, agitation, diaphoresis, seizures and coma).
Cardiovascular: Orthostatic hypotension, Syncope, Tachycardia.
Central Nervous System: Abnormal gait, Amnesia, Cognitive dysfunction, Depression, Difficulty in concentration, Hallucinations, Paresthesia, Seizure (see WARNINGS), Tremor.
Respiratory: Dyspnea.
Skin: Stevens-Johnson syndrome/Toxic epidermal necrolysis, Urticaria, Vesicles.
Special Senses: Dysgeusia.
Urogenital: Dysuria, Menstrual disorder.
Other adverse experiences, causal relationship unknown: A variety of other adverse events were reported infrequently in patients taking tramadol hydrochloride tablets during clinical trials and/or reported in post-marketing experience. A causal relationship between tramadol hydrochloride tablets and these events has not been determined. However, the most significant events are listed below as alerting information to the physician.
Cardiovascular: Abnormal ECG, Hypertension, Hypotension, Myocardial ischemia, Palpitations, Pulmonary edema, Pulmonary embolism.
Central Nervous System: Migraine, Speech disorders.
Gastrointestinal: Gastrointestinal bleeding, Hepatitis, Stomatitis, Liver failure.
Laboratory Abnormalities: Creatinine increase, Elevated liver enzymes, Hemoglobin decrease, Proteinuria.
Sensory: Cataracts, Deafness, Tinnitus.
DRUG ABUSE AND DEPENDENCE
Abuse
Tramadol has mu-opioid agonist activity. Tramadol Hydrochloride Tablet can be abused and may be subject to criminal diversion.
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. Drug addiction is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, use for non-medical purposes, and continued use despite harm or risk of harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
"Drug-seeking" behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated "loss" of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). "Doctor shopping" to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of tramadol hydrochloride tablet can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Concerns about abuse and addiction should not prevent the proper management of pain. However all patients treated with opioids require careful monitoring for signs of abuse and addiction, because use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
Proper assessment of the patient and periodic re-evaluation of therapy are appropriate measures that help to limit the potential abuse of this product.
Tramadol hydrochloride tablet is intended for oral use only.
Dependence
Tolerance is the need for increasing doses of drugs to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist (see also WARNINGS, Withdrawal).
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
Generally, tolerance and/or withdrawal are more likely to occur the longer a patient is on continuous therapy with tramadol hydrochloride tablet.
OVERDOSAGE
Acute overdosage with tramadol can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, seizures, bradycardia, hypotension, cardiac arrest, and death. Deaths due to overdose have been reported with abuse and misuse of tramadol (see WARNINGS, Misuse, Abuse, and Diversion). Review of case reports has indicated that the risk of fatal overdose is further increased when tramadol is abused concurrently with alcohol or other CNS depressants, including other opioids.
In the treatment of tramadol overdosage, primary attention should be given to the reestablishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
While naloxone will reverse some, but not all, symptoms caused by overdosage with tramadol, the risk of seizures is also increased with naloxone administration. In animals convulsions following the administration of toxic doses of tramadol hydrochloride tablet could be suppressed with barbiturates or benzodiazepines but were increased with naloxone. Naloxone administration did not change the lethality of an overdose in mice. Hemodialysis is not expected to be helpful in an overdose because it removes less than 7% of the administered dose in a 4-hour dialysis period.
DOSAGE AND ADMINISTRATION
Adults (17 years of age and over)
For patients with moderate to moderately severe chronic pain not requiring rapid onset of analgesic effect, the tolerability of tramadol hydrochloride tablets can be improved by initiating therapy with the following titration regimen: The total daily dose may be increased by 50 mg as tolerated every 3 days to reach 200 mg/day (50 mg q.i.d.). After titration, tramadol hydrochloride tablets 50 mg to 100 mg can be administered as needed for pain relief every four to six hours, not to exceed 400 mg per day.
For the subset of patients for whom rapid onset of analgesic effect is required and for whom the benefits outweigh the risk of discontinuation due to adverse events associated with higher initial doses, tramadol hydrochloride tablets 50 mg to 100 mg can be administered as needed for pain relief every four to six hours, not to exceed 400 mg per day.
Individualization of Dose
Good pain management practice dictates that the dose be individualized according to patient need using the lowest beneficial dose. Studies with tramadol in adults have shown that starting at the lowest possible dose and titrating upward will result in fewer discontinuations and increased tolerability.
- In all patients with creatinine clearance less than 30 mL/min, it is recommended that the dosing interval of tramadol hydrochloride tablets be increased to 12 hours, with a maximum daily dose of 200 mg. Since only 7% of an administered dose is removed by hemodialysis, dialysis patients can receive their regular dose on the day of dialysis.
- The recommended dose for adult patients with cirrhosis is 50 mg every 12 hours.
- In general, dose selection for an elderly patient over 65 years old should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy. For elderly patients over 75 years old, total dose should not exceed 300 mg/day.
HOW SUPPLIED
Tramadol hydrochloride tablet, 50 mg are available as white capsule shaped film coated tablets, debossed with “377” on one side and plain on the other side.
100's NDC 57664-377-08 Bottles of 100
500's NDC 57664-377-13 Bottles of 500
1000's NDC 57664-377-18 Bottles of 1000
Dispense in tight container.
Store at 25°C (77°F); excursions permitted to 15 - 30°C (59 -86°F).
Manufactured by:
Sun pharmaceutical industries
Dadra 396 191,India
Distributed by:
Caraco pharmaceutical Laboratories,Ltd
1150 Elijah McCoy Drive,Detroit,MI 48202
C.S.No:5243T64
Iss:08/10
| TRAMADOL HYDROCHLORIDE
tramadol hydrochloride tablet |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Lake Erie Medical DBA Quality Care Products LLC (831276758) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lake Erie Medical DBA Quality Care Products LLC | 831276758 | repack(49999-129) | |
