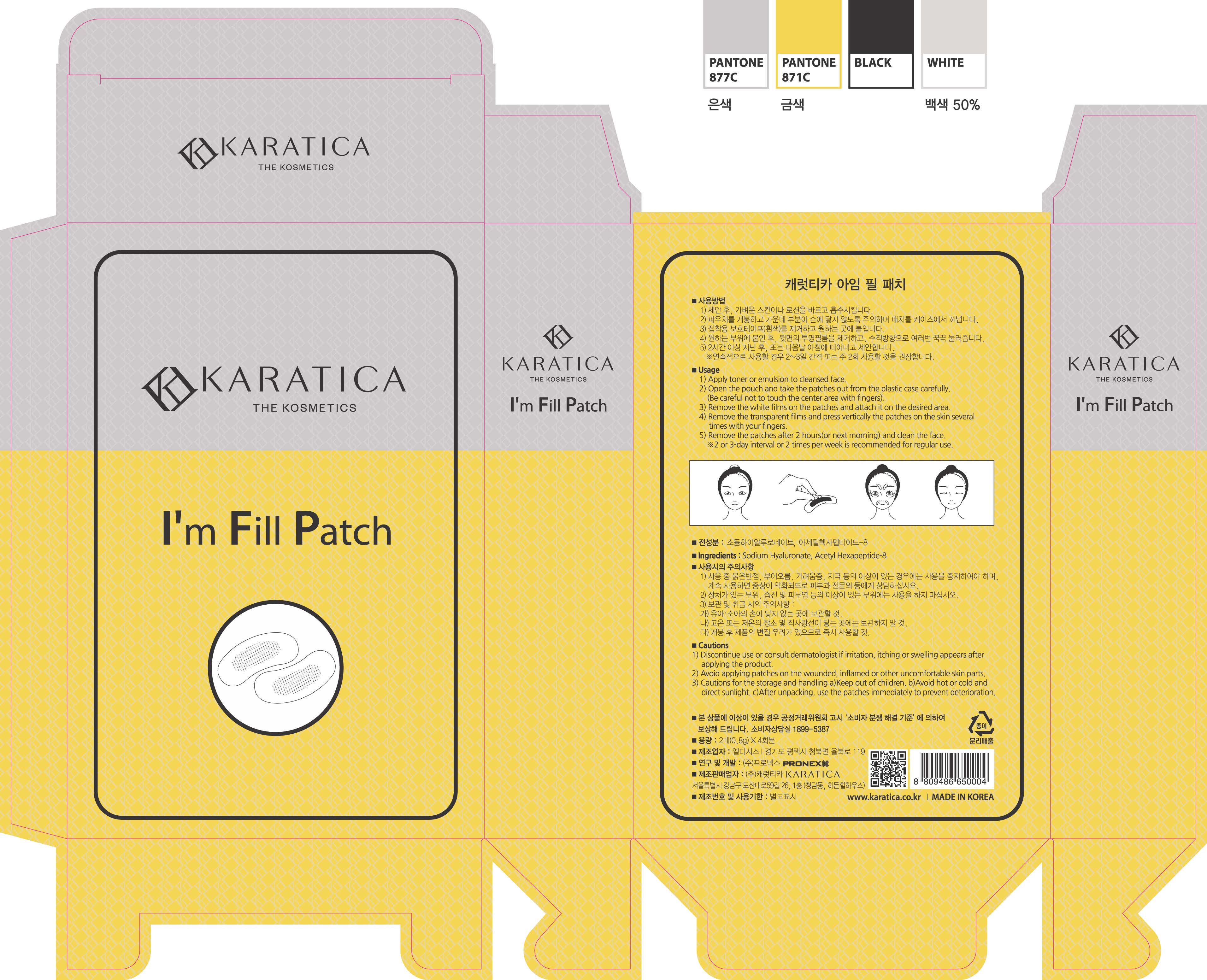
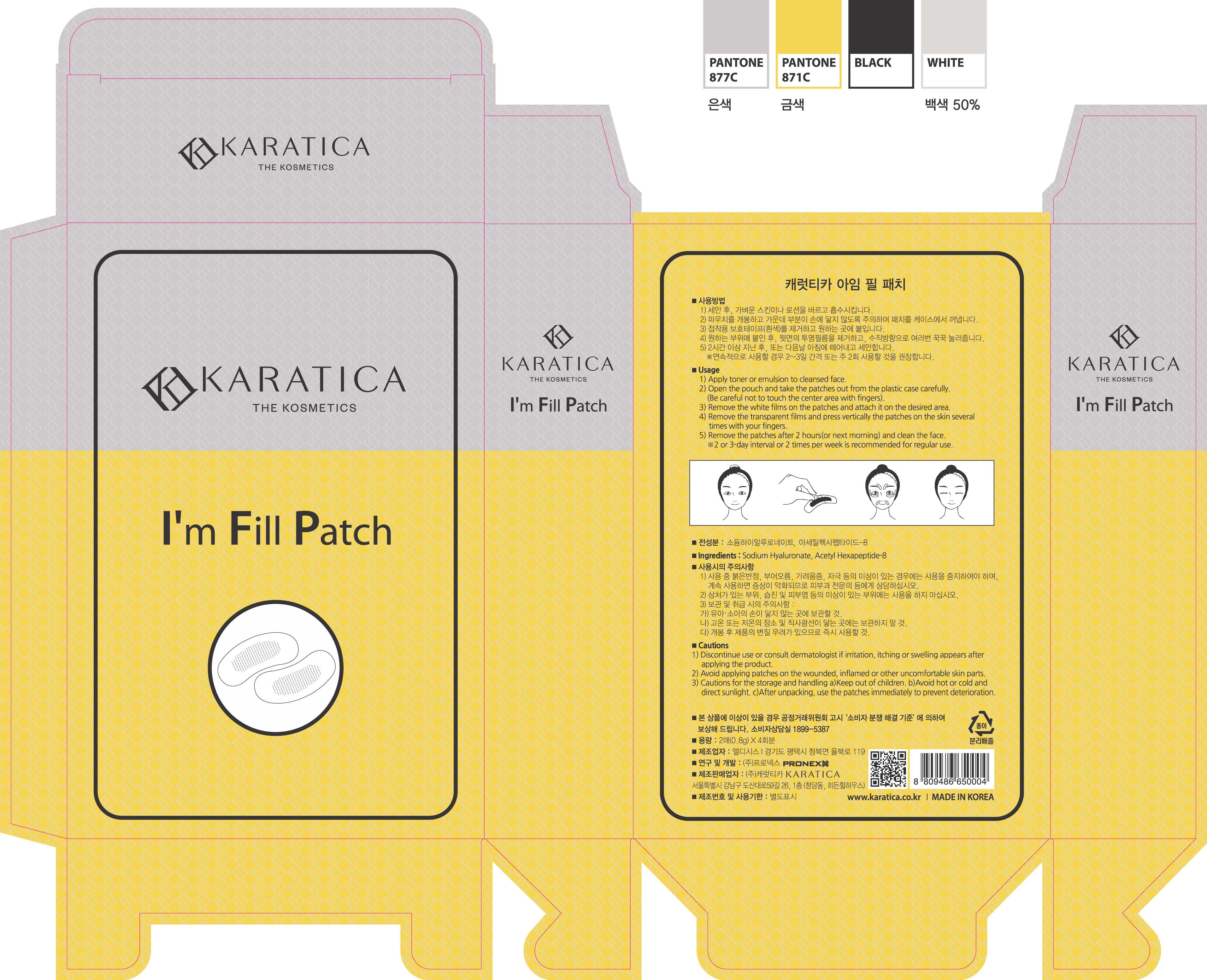
Label: IM FILL- hyaluronic acid patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 70514-0009-1 - Packager: Karatica Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 7, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
1) Apply toner or emulsion to cleansed face.
2) Open the pouch and take the patches out from the plastic case carefully.
(Be careful not to touch the center area with fingers.)
3) Remove the white films on the patches and attach it on the desired area.
4) Remove the transparent films and press vertically the patches on the skin several times with your fingers.
5) Remove the patches after 2 hours (or next morning) and clean the face.
*2 or 3 day interval or 2 times per week is recommended for regular use.
-
WARNINGS
1) Discontinue use or consult dermatologist if irritation, itching or swelling appears after applying the product.
2) Avoid applying patches on the wounded, inflamed or other uncomfortable skin part.
3) Cautions for the storage and handling a) Keep out of children. B) Avoid hot or cold and direct sunlight. c) After unpacking, use the patches immediately to prevent deterioration.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IM FILL
hyaluronic acid patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70514-0009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONATE SODIUM (UNII: YSE9PPT4TH) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONATE SODIUM 99.97 g in 100 g Inactive Ingredients Ingredient Name Strength ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70514-0009-1 4 in 1 PACKAGE 11/29/2017 1 0.8 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/29/2017 Labeler - Karatica Co., Ltd (689605545) Registrant - Karatica Co., Ltd (689605545) Establishment Name Address ID/FEI Business Operations Karatica Co., Ltd 689605545 manufacture(70514-0009)