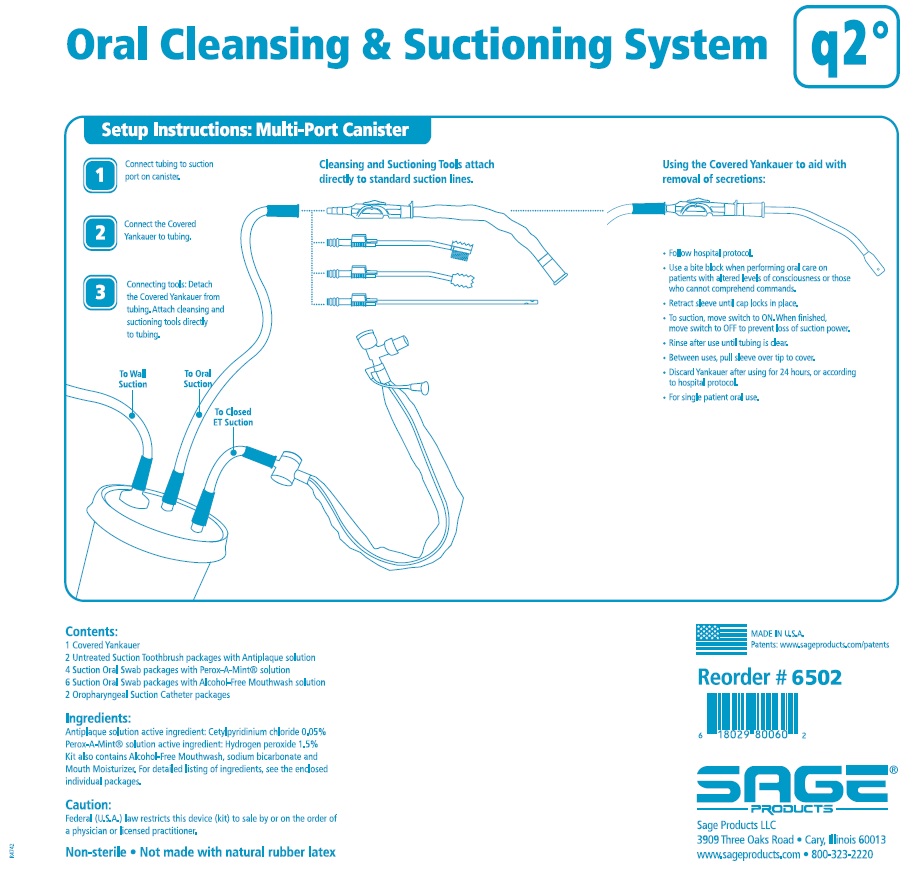
ORAL CLEANSING AND SUCTIONING SYSTEM, Q2- cetylpyridinium chloride and hydrogen peroxide
Sage Products LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
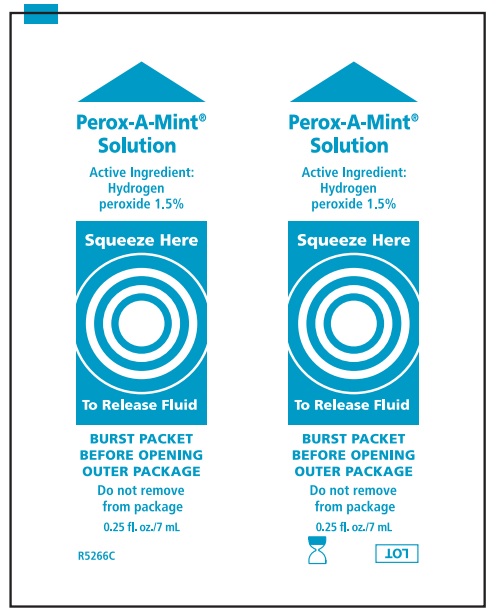
| Active Ingredient: PEROX-A-MINT:
| Purpose |
| Hydrogen Peroxide 1.5% | Oral Debriding Agent |
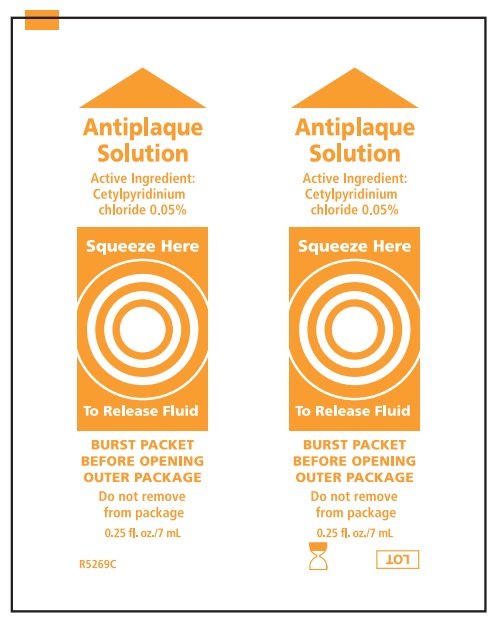
| Active Ingredient: ANTIPLAQUE*:
| Purpose |
| Cetylpyridinium chloride .05% | Antigingivitis/antiplaque |
USES
Suction Swab with Perox-A-Mint Solution:
- Aids in the removal of secretions and debris.
Suction Toothbrush with Antiplaque Solution
- Aids in the removal and prevention of plaque that leads to gingivitis.
WARNINGS
Suction Swab with Perox-A-Mint Solution:
Stop use and ask a doctor if:
- Sore mouth symptoms do not improve in 7 days.
- Swelling, rash or fever develops.
- Irritation, pain or redness persists or worsens.
Suction Toothbrush with Antiplaque Solution
Stop use and ask a dentist if
- Gingivitis, bleeding or redness persists for more than 2 weeks.
- You have painful or swollen gums, pus from the gum line, loose teeth or increasing spacing between the teeth. These may be signs or symptoms of periodontitis, a serious form of gum disease.
Suction Swab with Perox-A-Mint Solution:
Keep out of the reach of children.
If more than used for debriding is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Suction Toothbrush with Antiplaque Solution:
Keep out of reach of children under 6 years of age
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Suction Swab with Perox-A-Mint Solution:
-
Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Remove Mouth Moisturizer and Applicator Swab.
- Attach Suction Swab to suction line.
- Clean teeth and oral cavity for approximately one minute.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Swab. Reattach Covered Yankauer to suction line.
- Place Mouth Moisturizer on Applicator Swab.
- Apply as needed to lips and inside mouth.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Suction Toothbrush with Antiplaque Solution
-
Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Remove Mouth Moisturizer and Applicator Swab.
- Attach Toothbrush to suction line.
- Clean teeth and oral cavity for approximately one minute.
- To suction, place thumb over port.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Toothbrush. Reattach Covered Yankauer to suction line.
- Place Mouth Moisturizer on Applicator Swab.
- Apply as needed to lips and inside mouth.
- Adults and children 12 years of age and older: use two times daily or as directed by a dentist. Do not swallow the solution.
- Children ages 6 to 12 years: supervise use.
- Children under 6 years of age: do not use.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Inactive Ingredients
Suction Swab with Perox-A-Mint Solution:
Water, menthol flavor, polysorbate 80, phosphoric acid, sodium saccharin, Blue 1 (CI 42090), Yellow 6 (CI 15985)
Suction Toothbrush with Antiplaque Solution:
Water, sorbitol, peppermint flavor, potassium sorbate, polysorbate 80, polysorbate 20, citric acid, Blue 1 (CI42090)
Questions?
Call toll-free 800–323–2220
Manufactured for Sage Products LLC Cary, IL
NOT MADE WITH NATURAL RUBBER LATEX. SINGLE USE ONLY. MADE IN U.S.A. NON-STERILE.
Oral Cleansing and Suctioning System, Q2

Alcohol-Free Mouthwash, Antiplaque Solution, Perox-A-Mint