COMFORTCAINE TOPICAL ANESTHETIC- benzocaine gel
Mycone Dental Supply Co., Inc DBA Keystone Industries and Deepak Products Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Comfortcaine Cherry
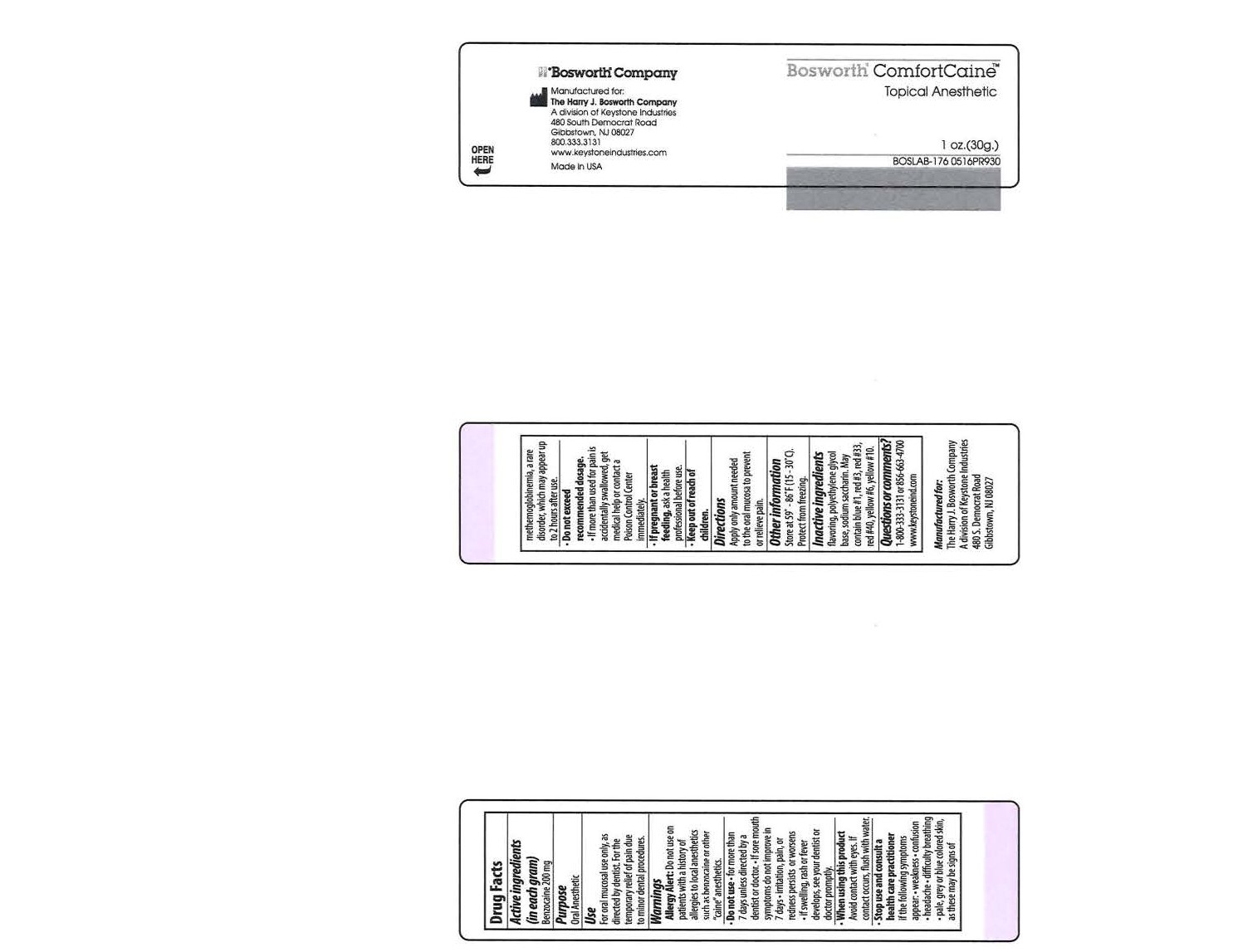
Uses
For oral mucosal use only, as directed by dentist. For the temporary relief of pain due to minor dental procedures.
Warnings
Allergy Alert: Do not use on patients with a history of allergies to local anesthetics such as benzocaine or other "caine" anesthetics.
Do not use
- for more than 7 days unless directed by a dentist or doctor.
- If sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- if swelling, rash or fever develops, see your dentist or doctor promptly.
Stop use and consult a health care practitioner
If the following symptoms appear: weakness, confusion, headache, difficulty breathing and/or pale, gray, or blue coloured skin, as these may be signs of methemoglobinemia, a rare disorder, which may appear up to 2 hours after use.
Do not exceed recommended dosage.
- If more than used for pain is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
Inactive ingredients
Flavoring, polyethylene glycol base, sodium saccharin. May contain blue #1, red #3, red #33, red #40, yellow #6, yellow #10.
| COMFORTCAINE
TOPICAL ANESTHETIC
benzocaine gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Mycone Dental Supply Co., Inc DBA Keystone Industries and Deepak Products Inc (014769301) |