LOVLUV VIVACITY BRIGHT CUSHION 21G- titanium dioxide, octinoxate, zinc oxide, octisalate liquid
Cnt Dream. Co., Ltd
----------
LOVLUV Vivacity Bright cushion 21 SPF50+ PA+++
Moist Natural
OTC – ACTIVE INGREIDENT SECTION
Titanium Dioxide, Ethylhexyl Methoxycinnamate, Zinc Oxide, Ethylhexyl Salicylate
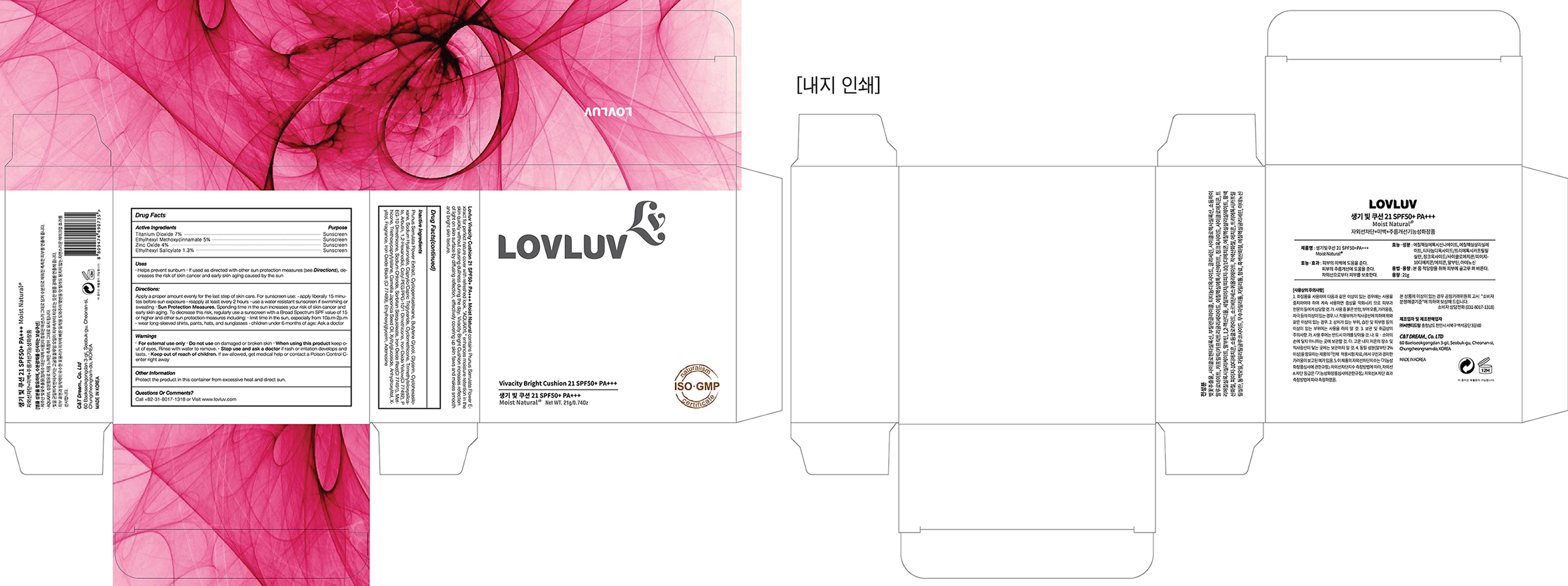
INACTIVE INGREDIENT SECTION

Prunus Serrulata Flower Extract ,Cyclopentasiloxane,Butylene Glycol, Glycerin,
Cyclohexasiloxane,Sodium Hyaluronate,Caprylic/Capric Triglyceride,Cyclomethicone,
Trimethylsiloxysilicate,Arbutin,1,2-Hexanediol,Cetyl PEG/PPG-10/1 Dimethicone,
Iron Oxide Yellow(CI 77492),PEG-10 Dimethicone,Sodium Chloride,Sorbitan Sesquioleate,
Iron Oxide Red(CI 77491),Methicone,Triethoxycaprylylsilane,Camellia Japonica Seed Oil,
Xylitylglucoside,Anhydroxylitol,Xylitol,Fragrance,Iron Oxide Black (CI 77499),
Ethylhexylglycerin,Adenosine
OTC – KEEP OUT OF REACH OF CHILDREN SECTION

*Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
WARNINGS SECTION

Warnings
*For external use only
*Do not use on damaged or broken skin
*When using this product keep out of eyes, Rinse with water to remove.
*Stop use and ask a doctor if rash or irritation develops and lasts.
*Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
INDICATIONS & USAGE SECTION

Uses
*helps prevent sunburn
*if used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions:
Apply a proper amount evenly for the last step of skin care.
For sunscreen use:
*apply liberally 15 minutes before sun exposure
*reapply at least every 2 hours
*use a water resistant sunscreen if swimming or sweating
*Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
*limit time in the sun, especially from 10a.m-2p.m
*wear long-sleeved shirts, pants, hats, and sunglasses
*children under 6 months of age: Ask a doctor
| LOVLUV VIVACITY BRIGHT CUSHION 21G
titanium dioxide, octinoxate, zinc oxide, octisalate liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
 |
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cnt Dream. Co., Ltd (694699750) |
| Registrant - Cnt Dream. Co., Ltd (694699750) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cnt Dream. Co., Ltd | 694699750 | manufacture(71909-0735) | |