ZOTEX-C- codeine phosphate, phenylephrine hydrochloride and pyrilamine maleate syrup
Vertical Pharmaceuticals, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ZOTEX™-C Syrup
CV
DESCRIPTION
Zotex™-C Syrup is an antitussive decongestant and antihistamine available for oral administration as a syrup.
Each 5 mL (1 teaspoonful) of red-colored, cherry-flavored syrup contains:
| Codeine Phosphate (WARNING-May be habit forming) | 10 mg |
| Phenylephrine Hydrochloride | 5 mg |
| Pyrilamine Maleate | 5 mg |
Inactive Ingredients: Glycerin, Propylene Glycol, Sorbitol, Citric Acid, Sodium Citrate, Sodium Saccharin, FD&C Red #40, Cherry Flavor, Purified Water.
Codeine Phosphate is an alkaloid, obtained from opium or prepared from morphine by methylation. Codeine phosphate occurs as fine, white, needle-shaped crystals, or white crystalline powder. It is affected by light. Its chemical name is Morphinan-6-ol, 7,8-didehydroxy-4,5-epoxy-3-methoxy-17-methyl- ,(5α,6α)-, phosphate (1:1) (salt), hemihydrate.
Its structure is as follows:
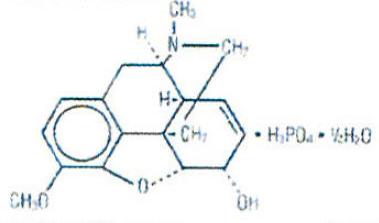
C12H21NO3 • H3PO4 • ½H2O M.W. 406.37
Phenylephrine Hydrochloride is a sympathomimatic amine decongestant which occurs as white or practically white, odorless crystals having a bitter taste. It is freely soluble in water and in alcohol. The chemical name is: Benzenemethanol, 3-hydroxy-α-[(methylamino)methyl]-hydrochloride (R)-. Its structure is as follows:
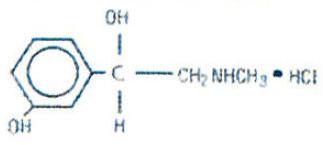
C9H13NO2 • HCl M.W. 203.67
Pyrilamine Maleate is an antihistamine having the chemical name 2-[(2-Dimethylamino)ethyl)(p-methoxybenzyl)amino] pyridine maleate (1:1). Its structural formula is as follows:
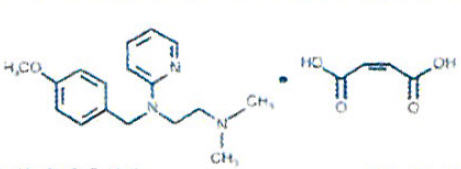
C17H23N3O•C4H4O4 MW 401.47
CLINICAL PHARMACOLOGY
Codeine Phosphate is a centrally acting analgesic and antitussive which is well absorbed orally. Following absorption, codeine is metabolized by the liver and metabolic products are excreted in the urine.
Phenylephrine Hydrochloride is a sympathomimetic that acts predominantly on alpha receptors and has little action on beta receptors. Phylephrine Hydrochloride causes constriction of blood vessels, which shrinks swollen mucous membranes, reduces tissue hyperemia edema, nasal congestion and increases nasal airway patency. It, therefore, functions as an oral nasal decongestant while causing minimal central nervous system stimulation.
Pyrilamine Maleate is an antihistamine used in suppressing symptoms of allergic rhinitis. However, it is more prone to cause drowsiness than some other antihistamines.
INDICATIONS AND USAGE
Zotex™-C Syrup is indicated for symptomatic relief of coughs and upper respiratory symptoms. Including nasal congestion, associated the common cold.
CONTRAINDICATIONS
Patients with hypersensitivity or idiosyncrasy to any of its ingredients. Do not use in newborn infants, premature infants, in nursing mothers, in patients with severe hypertension, severe coronary artery disease, ischemic heat disease, or in those receiving monoamine oxidase (MAO) inhibitors. Antihistamines are contraindicated in patients with narrow-angle glaucoma, urinary retention, peptic ulcer, and during an asthma attack. Antihistamines should not be used to treat lower respiratory tract conditions including asthma.
WARNINGS
Do not exceed the recommended dosage. Patients with persistent cough such as occur with smoking, asthma, emphysema or where cough is accompanied by excessive secretions should not take this product. A persistent cough may be a sign of a serious condition. If the cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor. Use caution when giving to children or patients with chronic pulmonary disease, shortness of breath, difficulty in breathing, asthma, emphysema, high blood pressure, heart disease, diabetes, thyroid disease or difficulty in urination due to enlargement of the prostate gland unless directed by a physician. Antihistamines may cause hyperexcitability, especially in children. At doses higher than the recommended dose, nervousness, dizziness or sleeplessness may occur. Especially in infants and small children, antihistamines in overdosage may cause hallucinations, convulsions, and death.
If a hypertensive crisis occurs, these drugs should be discontiued immediately and therapy to lower blood pressure should be instituted immediately. Fever should be managed by means of external cooling.
PRECAUTIONS
General
Before prescribing medication to suppress or modify cough, it is important to ascertain that the underlying cause of cough is identified, that modification of cough does not increase the risk of clinical or physiologic complications, and that appropriate therapy for the primary disease is provided. Because of its sympathomimetic component, Zotex™-C Syrup should be used with caution in patients with diabetes mellitus, hypertension, heart disease, or thyroid disease.
Information for Patients
Patients should be warned about engaging in activities requiring mental alertness and motor coordination, such as driving a car or operating machinery. Patients should be cautioned to get up slowly from a lying or sitting position and to lie down if nausea occurs.
Drug Interactions
Patients receiving other narcotic analgesics, anti-psychotics, anti-anxiety agents, or other CNS depressants (including alcohol) concomitantly with this drug may exhibit an additive CNS depression. When such combined therapy is contemplated, the dose of one or both agents should be reduced. The concurrent use of anticholinergics with codeine may produce paralytic ileus. MAO inhibitors prolong and intensify the anticholinergic effects of antihistamines. Antihistamines may have additive effects with alcohol and other CNS depressants, e.g.; hypnotics, sedatives, tranquilizers, antianxiety agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies of Zotex™-C Syrup to assess the carcinogenic and mutagenic potential to the effect on fertility have not been performed.
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Zotex™-C Syrup. It is also not known whether Zotex™-C Syrup can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Zotex™-C Syrup should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether codeine is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from this product, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Because of the higher risk of intolerance of antihistamines in small infants generally, and in newborns and prematures in particular. Zotex™-C Syrup is contraindicated in nusing mothers.
ADVERSE REACTIONS
The most frequent adverse reactions to Zotex™-C Syrup include sedation; dryness of mouth, nose, and throat; thickening of bronchial secretions; dizziness. Other adverse reactions may include:
General: urinary, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose, and throat.
Cardiovascular System: hypotension, headache, palpitations, tachycardia, extra systoles.
Dermatologic: urticaria, drug rash, photosensitivity, and pruritus.
Central Nervous System: sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesias, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, peuritus convulsions.
Gastrointestinal: epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation.
Genitourinary: urinary frequency, difficult urination, urinary retention, early mensea.
Respiratory: thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
Hematologic System: hemolytic anemia, thrombocytopenia, agranulocytosis.
OVERDOSAGE
Signs and Symptoms
Overdosage of Phenylephrine Hydrochloride may be associated with central nervous system stimulation, tachycardia, hypertension, and cardiac arrhythmias.
Toxic Doses
Treatment
Induce emesis if patient is alert and is seen prior to 6 hours following ingestion. Precautions against aspiration must be taken, especially in infants and small children. Gastric lavage may be carried out although in some instances tracheotomy may be necessary prior to lavage. CNS stimulants may counter CNS depression. Should CNS hyperactivity or convulsive seizures occur, intravenous short-acting barbiturates may be indicated. Hypertensive responses and/or tachycardia should be treated appropriately. Oxygen, intravenous fluids, and other supportive measures should be employed as indicated.
DOSAGE AND ADMINISTRATION1
Adults and Children 12 years of age and older: 1-2 teaspoonfuls every 4-6 hours, not to exceed 12 teaspoonfuls in a 24 hour period.
Children 6 to 12 years of age: 1/2 to 1 teaspoonful every 4-6 hours, not to exceed 6 teaspoonfuls in a 24 hour period.
- 1
- In mild cases or in particularly sensitive patients, less frequent or reduced doses may be adequate.
HOW SUPPLIED
Zotex™-C Syrup is a sugar free, alcohol free, cherry-flavored, red-colored syrup in 16 fl oz (473 mL) bottles.
NDC 68025-037-16 and professional samples of 10 mL NDC 68025-037-10.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
PRINCIPAL DISPLAY PANEL - 473 mL Bottle
NDC 68025-037-16
ZOTEX™-C
Syrup
Antitussive / Decongestant / Antihistamine
Cherry Flavor
Each teaspoonful (5 mL) for oral
administration contains:
| Codeine Phosphate* | 10 mg |
| *WARNING: May be habit-forming. | |
| Phenylephrine HCl | 5 mg |
| Pyrilamine Maleate | 5 mg |
Alcohol Free • Sugar Free
Rx Only
VERTICAL
PHARMACEUTICALS, INC.
16 fl oz (473 mL)

| ZOTEX-C
codeine phosphate, phenylephrine hydrochloride and pyrilamine maleate syrup |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Vertical Pharmaceuticals, Inc. (173169017) |