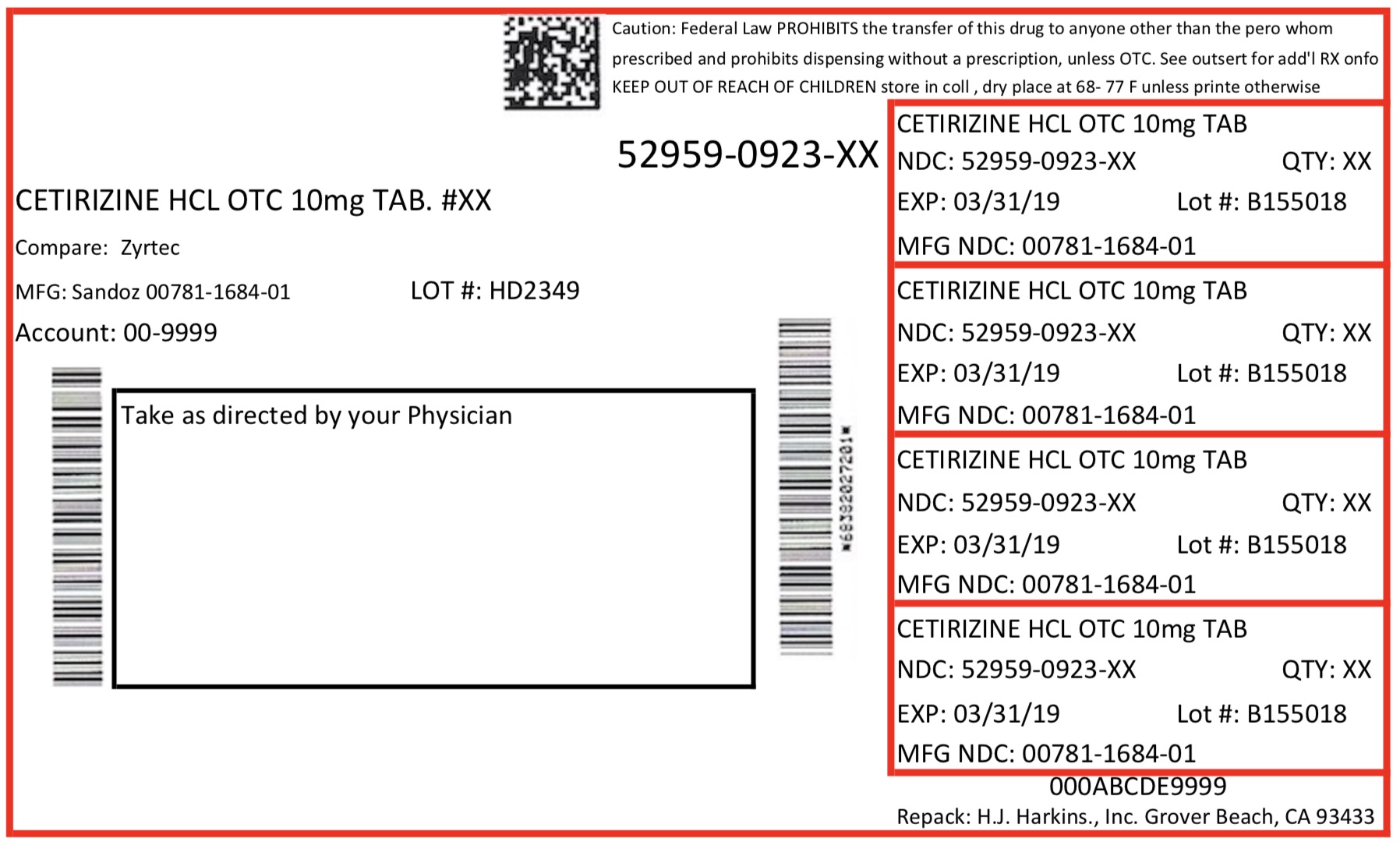
Label: CETIRIZINE HYDROCHLORIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 52959-923-07, 52959-923-10, 52959-923-14, 52959-923-15, view more52959-923-20, 52959-923-30, 52959-923-60, 52959-923-90 - Packager: H. J. Harkins Company Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 1, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Keep out of Reach of Children
- USES
-
Warnings
antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
When using this product
•
drowsiness may occur
•
avoid alcoholic drinks
•
alcohol, sedatives, and tranquilizers may increase drowsiness
•
be careful when driving a motor vehicle or operating machineryStop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding:
•
if breast-feeding: not recommended
•
if pregnant: ask a health professional before use.Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
-
Directions
dults and children 6 years and over
One 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms.
adults 65 years and over
ask a doctor
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
- Inactive Ingredient
- Package Label. Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52959-923 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Product Characteristics Color white Score score with uneven pieces Shape ROUND Size 8mm Flavor Imprint Code SZ;906 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52959-923-10 10 in 1 CONTAINER; Type 0: Not a Combination Product 01/03/2017 2 NDC:52959-923-15 15 in 1 CONTAINER; Type 0: Not a Combination Product 01/03/2017 3 NDC:52959-923-30 30 in 1 CONTAINER; Type 0: Not a Combination Product 01/03/2017 4 NDC:52959-923-60 60 in 1 CONTAINER; Type 0: Not a Combination Product 01/03/2017 5 NDC:52959-923-90 90 in 1 CONTAINER; Type 0: Not a Combination Product 01/03/2017 6 NDC:52959-923-07 7 in 1 CONTAINER; Type 0: Not a Combination Product 01/03/2017 7 NDC:52959-923-20 20 in 1 CONTAINER; Type 0: Not a Combination Product 01/03/2017 8 NDC:52959-923-14 14 in 1 CONTAINER; Type 0: Not a Combination Product 01/03/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077946 01/03/2017 Labeler - H. J. Harkins Company Inc. (147681894) Establishment Name Address ID/FEI Business Operations H. J. Harkins Company Inc. 147681894 manufacture(52959-923) , relabel(52959-923) , repack(52959-923)