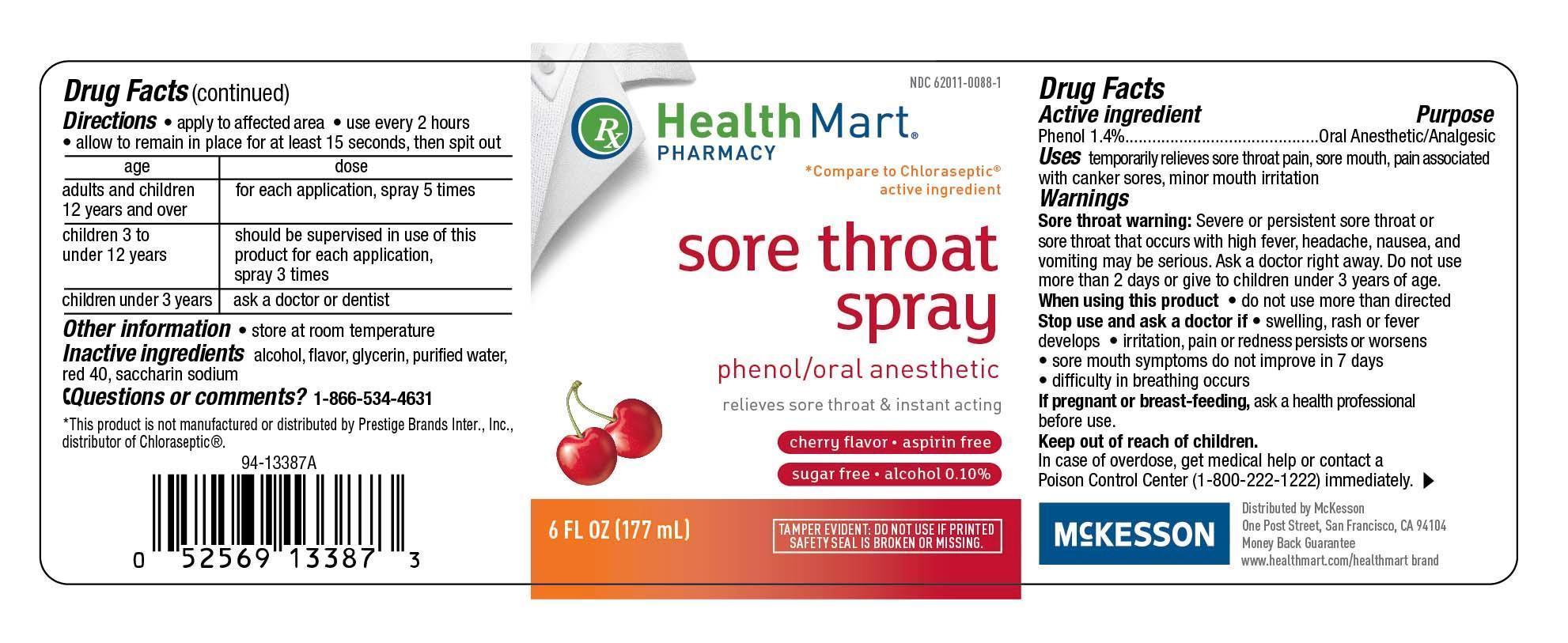
SORE THROAT CHERRY- phenol liquid
McKesson (Health Mart)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Use(s)
temporarily relieves sore throat pain, sore mouth, pain associated with canker sores, minor mouth irritation
Warnings
Sore Throat Warning: Severe or persistent sore throat or sore throat that occurs with high fever, headache, nausea, and vomiting may be serious. Ask a doctor right away. Do not use more than 2 days or give to children under 3 years of age.
Stop use and ask doctor if
- irritation, pain or redness persists or worsens
- sore mouth symptoms do not improve in 7 days
- swelling, rash or fever develops
- difficulty in breathing occurs
Keep Out of Reach of Children
Keep this and all drugs out of the reach of children.In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Directions
apply to affected areause every 2 hoursallow to remain in place for at least 15 seconds, then spit out
AgeDose adults and children's 12 years or older..............for each application, spray 5 times children 3 years to 12 years................................should be supervised in use of this product for each application, spray 3 times children under 3 years.........................................ask a doctor or dentist
Principal Display Panel
Health Mart Pharmacy
compare to Chloraseptic active ingredient
Sore throat spray
phenol/oral anesthetic
Relieves fore throat and instant acting
cherry flavor aspirin free
sugar free alcohol 0.10 percent
6 fl oz 177mL
Tamper evident do not use if printed safety seal is broken or missing.
| SORE THROAT
CHERRY
phenol liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - McKesson (Health Mart) (177667227) |