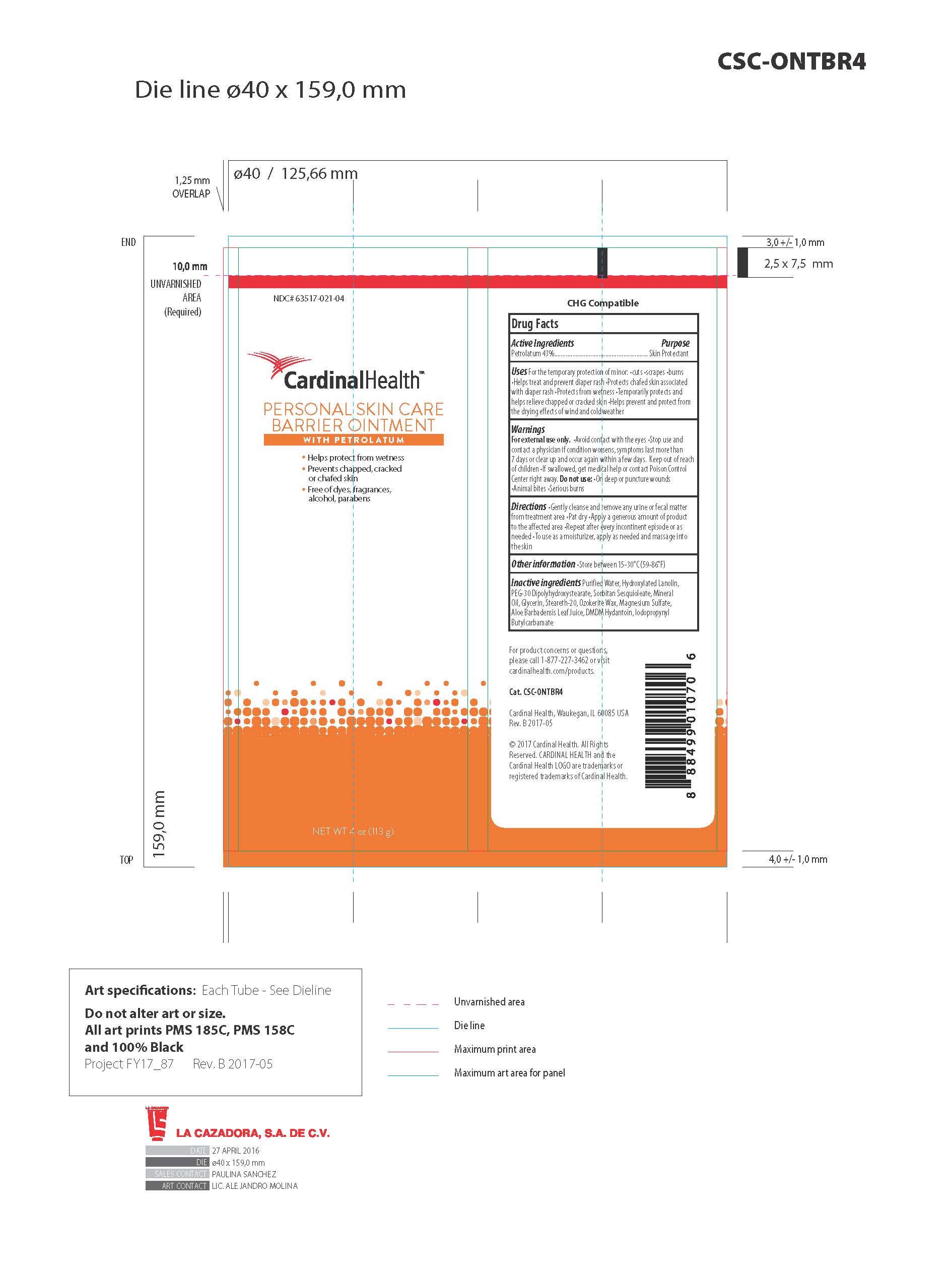
BARRIER SKIN PROTECTANT- 43% petrolatum skin protectant cream
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cardinal Barrier Ointment
Warnings
- For external use only
- Avoid contact with the eyes
- Stop use and contact a physician if conditions worsens, symptoms last more than 7 days or clear up and occur again within a few days
- Keep out of reach of children
- If swallowed, get medical help or contact Poison Control Center right away
- Do not use on deep or puncture wounds, animal bites or serious burns
Uses
- For the temporary protection of: diaper rash, minor cuts, scrapes and burns
- Temporarily protects and helps relieve chapped or cracked skin
- Helps protect from the drying effects of wind and cold weather
Directions
- Gently cleanse and remove any urine or fecal matter from treatment area
- Pat dry
- Apply generous amount of product to the affected area
- Repeat after every incontinent episode or as needed
- To use as a moisturizer, apply liberally and massage into skin
| BARRIER SKIN PROTECTANT
43% petrolatum skin protectant cream |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Cardinal Health (961027315) |
Revised: 11/2022
Document Id: 54481e88-b8c8-4ddc-b82a-8cac3384991f
Set id: 5e6e6d22-611f-4b83-b0f2-c2bd2467b318
Version: 6
Effective Time: 20221114
Cardinal Health