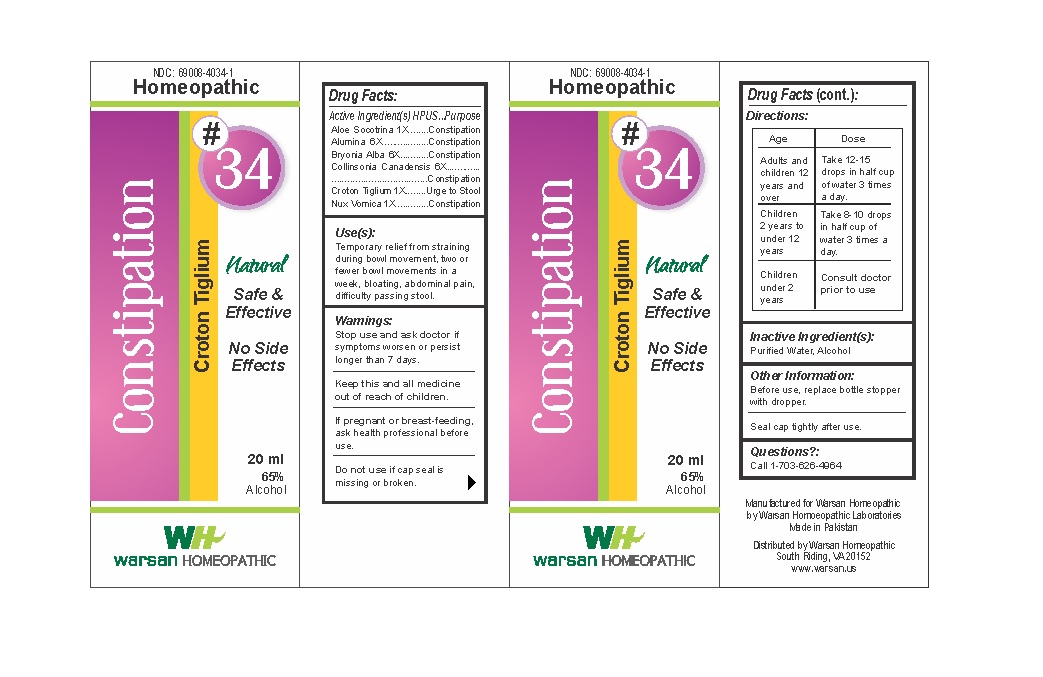
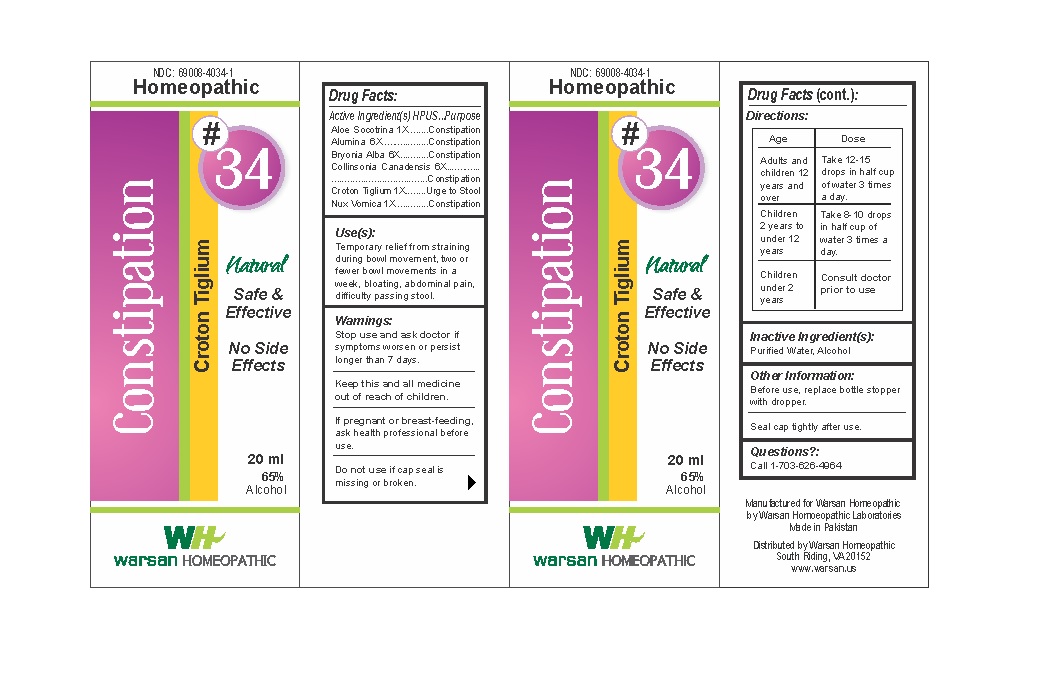
Label: COMBINATION REMEDY 34 - CONSTIPATION- aloe socotrina, alumina, bryonia alba, collinsonia canadensis, croton tiglium, nux vomica solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 69008-4034-1 - Packager: Warsan Homoeopathic Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 25, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- ACTIVE INGREDIENT
- PURPOSE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COMBINATION REMEDY 34 - CONSTIPATION
aloe socotrina, alumina, bryonia alba, collinsonia canadensis, croton tiglium, nux vomica solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69008-4034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 1 mL COLLINSONIA CANADENSIS ROOT (UNII: O2630F3XDR) (COLLINSONIA CANADENSIS ROOT - UNII:O2630F3XDR) COLLINSONIA CANADENSIS ROOT 6 [hp_X] in 1 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) (ALOE VERA LEAF - UNII:ZY81Z83H0X) ALOE VERA LEAF 1 [hp_X] in 1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 1 [hp_X] in 1 mL ALUMINUM OXIDE (UNII: LMI26O6933) (ALUMINUM OXIDE - UNII:LMI26O6933) ALUMINUM OXIDE 6 [hp_X] in 1 mL CROTON TIGLIUM SEED (UNII: 0HK2GZK66E) (CROTON TIGLIUM SEED - UNII:0HK2GZK66E) CROTON TIGLIUM SEED 1 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69008-4034-1 20 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 11/16/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/15/2017 Labeler - Warsan Homoeopathic Laboratories (645579772) Registrant - Warsan Homoeopathic Laboratories (645579772) Establishment Name Address ID/FEI Business Operations Warsan Homoeopathic Laboratories 645579772 manufacture(69008-4034) , pack(69008-4034) , label(69008-4034)