PRILOXX LP EXTERNAL KIT- lidocaine and prilocaine cream
Sircle Laboratories LLC
----------
Priloxx LP External Kit
DESCRIPTION
Lidocaine 2.5% and Prilocaine 2.5% Cream, USP is an emulsion in which the oil phase is a eutectic
mixture of lidocaine and prilocaine cream in a ratio of 1:1 by weight. This eutectic mixture has a
melting point below room temperature and therefore both local anesthetics exist as a liquid oil rather
than as crystals. It is packaged in 5 gram and 30 gram tubes.
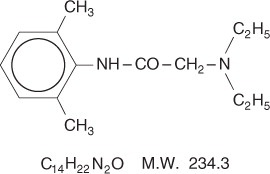
Lidocaine is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), has an
octanol: water partition ratio of 43 at pH 7.4, and has the following structure:
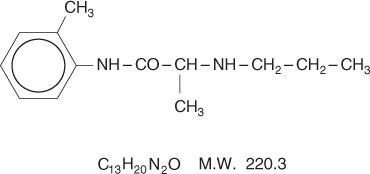
Prilocaine is chemically designated as propanamide, N-(2-methylphenyl)-2-(propylamino), has an
octanol: water partition ratio of 25 at pH 7.4, and has the following structure:
Each gram of lidocaine and prilocaine cream contains lidocaine 25 mg, prilocaine 25 mg,
polyoxyethylene fatty acid esters (as emulsifiers), carboxypolymethylene (as a thickening agent),
sodium hydroxide to adjust to a pH approximating 9, and purified water to 1 gram. Lidocaine and
prilocaine cream contains no preservative, however it passes the USP antimicrobial effectiveness test
due to the pH. The specific gravity of lidocaine and prilocaine cream is 1.00.
CLINICAL PHARMACOLOGY
Mechanism of Action: Lidocaine and prilocaine cream applied to intact skin under occlusive dressing,
provides dermal analgesia by the release of lidocaine and prilocaine from the cream into the epidermal
and dermal layers of the skin and by the accumulation of lidocaine and prilocaine in the vicinity of
dermal pain receptors and nerve endings. Lidocaine and prilocaine are amide-type local anesthetic
agents. Both lidocaine and prilocaine stabilize neuronal membranes by inhibiting the ionic fluxes
required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
The onset, depth and duration of dermal analgesia on intact skin provided by lidocaine and prilocaine
cream depend primarily on the duration of application. To provide sufficient analgesia for clinical
procedures such as intravenous catheter placement and venipuncture, lidocaine and prilocaine cream
should be applied under an occlusive dressing for at least 1 hour. To provide dermal analgesia for
clinical procedures such as split skin graft harvesting, lidocaine and prilocaine cream should be applied
under occlusive dressing for at least 2 hours. Satisfactory dermal analgesia is achieved 1 hour after
application, reaches maximum at 2 to 3 hours, and persists for 1 to 2 hours after removal. Absorption
from the genital mucosa is more rapid and onset time is shorter (5 to 10 minutes) than after application to
intact skin. After a 5 to 10 minute application of lidocaine and prilocaine cream to female genital
mucosa, the average duration of effective analgesia to an argon laser stimulus (which produced a sharp,
pricking pain) was 15 to 20 minutes (individual variations in the range of 5 to 45 minutes).
Dermal application of lidocaine and prilocaine cream may cause a transient, local blanching followed by
a transient, local redness or erythema.
Pharmacokinetics: Lidocaine and prilocaine cream is a eutectic mixture of lidocaine 2.5% and
prilocaine 2.5% formulated as an oil in water emulsion. In this eutectic mixture, both anesthetics are
liquid at room temperature (see DESCRIPTION) and the penetration and subsequent systemic absorption
of both prilocaine and lidocaine are enhanced over that which would be seen if each component in
crystalline form was applied separately as a 2.5% topical cream.
Absorption: The amount of lidocaine and prilocaine systemically absorbed from lidocaine and prilocaine
cream is directly related to both the duration of application and to the area over which it is applied. In
two pharmacokinetic studies, 60 g of lidocaine and prilocaine cream (1.5 g lidocaine and 1.5 g
prilocaine) was applied to 400 cm of intact skin on the lateral thigh and then covered by an occlusive
dressing. The subjects were then randomized such that one-half of the subjects had the occlusive
dressing and residual cream removed after 3 hours, while the remainder left the dressing in place for 24
hours. The results from these studies are summarized below.
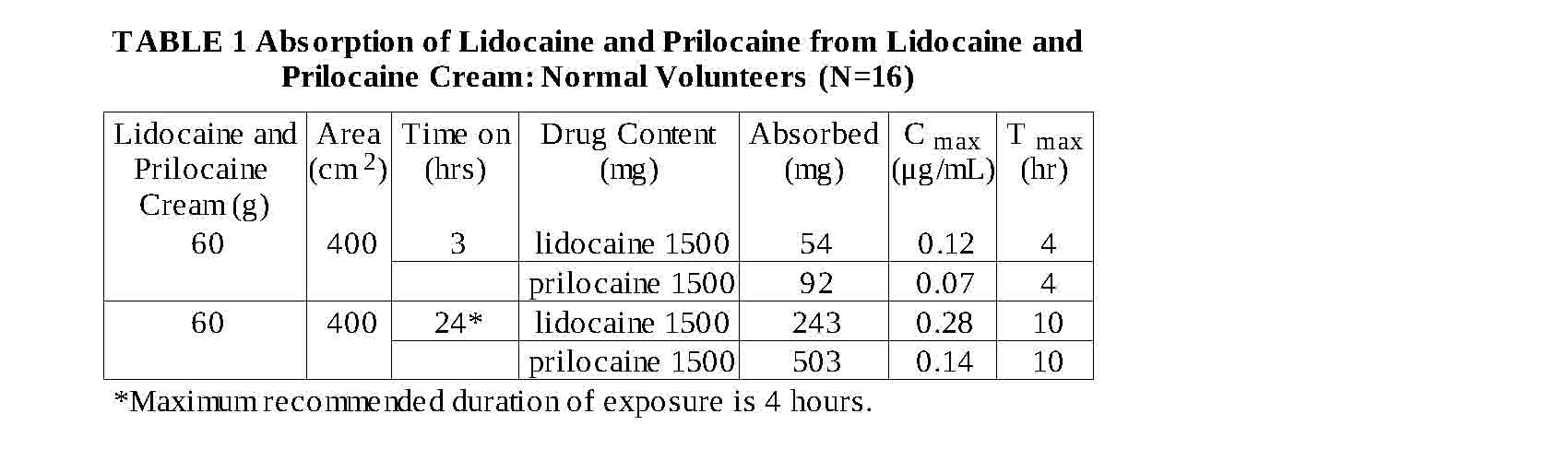
When 60 g of lidocaine and prilocaine cream was applied over 400 cm for 24 hours, peak blood
levels of lidocaine are approximately 1/20 the systemic toxic level. Likewise, the maximum prilocaine
level is about 1/36 the toxic level. In a pharmacokinetic study, lidocaine and prilocaine cream was
applied to penile skin in 20 adult male patients in doses ranging from 0.5 g to 3.3 g for 15 minutes.
Plasma concentrations of lidocaine and prilocaine following lidocaine and prilocaine cream application
in this study were consistently low (2.5 to 16 ng/mL for lidocaine and 2.5 to 7 ng/mL for prilocaine).
The application of lidocaine and prilocaine cream to broken or inflamed skin, or to 2,000 cm or more
of skin where more of both anesthetics are absorbed, could result in higher plasma levels that could, in
susceptible individuals, produce a systemic pharmacologic response.
The absorption of lidocaine and prilocaine cream applied to genital mucous membranes was studied in
two open-label clinical trials. Twenty-nine patients received 10 g of lidocaine and prilocaine cream
applied for 10 to 60 minutes in the vaginal fornices. Plasma concentrations of lidocaine and prilocaine
following lidocaine and prilocaine cream application in these studies ranged from 148 to 641 ng/mL for
lidocaine and 40 to 346 ng/mL for prilocaine and time to reach maximum concentration (t ) ranged
from 21 to 125 minutes for lidocaine and from 21 to 95 minutes for prilocaine. These levels are well
below the concentrations anticipated to give rise to systemic toxicity (approximately 5000 ng/mL for
lidocaine and prilocaine).
Distribution: When each drug is administered intravenously, the steady-state volume of distribution is
1.1 to 2.1 L/kg (mean 1.5, ±0.3 SD, n=13) for lidocaine and is 0.7 to 4.4 L/kg (mean 2.6, ±1.3 SD, n=13)
for prilocaine. The larger distribution volume for prilocaine produces the lower plasma concentrations
of prilocaine observed when equal amounts of prilocaine and lidocaine are administered. At
concentrations produced by application of lidocaine and prilocaine cream, lidocaine is approximately
70% bound to plasma proteins, primarily alpha-1-acid glycoprotein. At much higher plasma
concentrations (1 to 4 μg/mL of free base) the plasma protein binding of lidocaine is concentration
dependent. Prilocaine is 55% bound to plasma proteins. Both lidocaine and prilocaine cross the
placental and blood brain barrier, presumably by passive diffusion.
Metabolism: It is not known if lidocaine or prilocaine are metabolized in the skin. Lidocaine is
metabolized rapidly by the liver to a number of metabolites including monoethylglycinexylidide
(MEGX) and glycinexylidide (GX), both of which have pharmacologic activity similar to, but less
potent than that of lidocaine. The metabolite, 2,6-xylidine, has unknown pharmacologic activity.
Following intravenous administration, MEGX and GX concentrations in serum range from 11 to 36% and
from 5 to 11% of lidocaine concentrations, respectively. Prilocaine is metabolized in both the liver and
kidneys by amidases to various metabolites including ortho-toluidine and N-n-propylalanine. It is not
metabolized by plasma esterases. The ortho-toluidine metabolite has been shown to be carcinogenic in
several animal models (see Carcinogenesis subsection of PRECAUTIONS). In addition, ortho-toluidine
can produce methemoglobinemia following systemic doses of prilocaine approximating 8 mg/kg (see
ADVERSE REACTIONS). Very young patients, patients with glucose-6-phosphate dehydro- genase
deficiencies and patients taking oxidizing drugs such as antimalarials and sulfonamides are more
susceptible to methemoglobinemia (seeMethemoglobinemia subsection of WARNINGS).
Elimination: The terminal elimination half-life of lidocaine from the plasma following IV administration
is approximately 65 to 150 minutes (mean 110, ±24 SD, n=13). More than 98% of an absorbed dose of
lidocaine can be recovered in the urine as metabolites or parent drug. The systemic clearance is 10 to
20 mL/min/kg (mean 13, ±3 SD, n=13). The elimination half-life of prilocaine is approximately 10 to 150
minutes (mean 70, ±48 SD, n=13). The systemic clearance is 18 to 64 mL/min/kg (mean 38, ±15 SD,
n=13). During intravenous studies, the elimination half-life of lidocaine was statistically significantly
longer in elderly patients (2.5 hours) than in younger patients (1.5 hours). No studies are available on the
intravenous pharmacokinetics of prilocaine in elderly patients.
Pediatrics: Some pharmacokinetic (PK) data are available in infants (1 month to <2 years old) and
children (2 to <12 years old). One PK study was conducted in 9 full-term neonates (mean age: 7 days and
mean gestational age: 38.8 weeks). The study results show that neonates had comparable plasma
lidocaine and prilocaine concentrations and blood methemoglobin concentrations as those found in
previous pediatric PK studies and clinical trials. There was a tendency towards an increase in
methemoglobin formation. However, due to assay limitations and very little amount of blood that could
be collected from neonates, large variations in the above reported concentrations were found.
Special Populations: No specific PK studies were conducted. The half-life may be increased in cardiac
or hepatic dysfunction. Prilocaine's half-life also may be increased in hepatic or renal dysfunction since
both of these organs are involved in prilocaine metabolism.
Special Populations: No specific PK studies were conducted. The half-life may be increased in cardiac
or hepatic dysfunction. Prilocaine's half-life also may be increased in hepatic or renal dysfunction since
both of these organs are involved in prilocaine metabolism.
CLINICAL STUDIES
Lidocaine and prilocaine cream application in adults prior to IV cannulation or venipuncture was studied
in 200 patients in four clinical studies in Europe. Application for at least 1 hour provided significantly
more dermal analgesia than placebo cream or ethyl chloride. Lidocaine and prilocaine cream was
comparable to subcutaneous lidocaine, but was less efficacious than intradermal lidocaine. Most
patients found lidocaine and prilocaine cream treatment preferable to lidocaine infiltration or ethyl
chloride spray.
Lidocaine and prilocaine cream was compared with 0.5% lidocaine infiltration prior to skin graft
harvesting in one open label study in 80 adult patients in England. Application of lidocaine and
prilocaine cream for 2 to 5 hours provided dermal analgesia comparable to lidocaine infiltration.
Lidocaine and prilocaine cream application in children was studied in seven non-US studies (320
patients) and one US study (100 patients). In controlled studies, application of lidocaine and prilocaine
cream for at least 1 hour with or without presurgical medication prior to needle insertion provided
significantly more pain reduction than placebo. In children under the age of seven years, lidocaine and
prilocaine cream was less effective than in older children or adults.
Lidocaine and prilocaine cream was compared with placebo in the laser treatment of facial port-wine
stains in 72 pediatric patients (ages 5 to 16). Lidocaine and prilocaine cream was effective in providing
pain relief during laser treatment.
Lidocaine and prilocaine cream alone was compared with lidocaine and prilocaine cream followed by
lidocaine infiltration and lidocaine infiltration alone prior to cryotherapy for the removal of male genital
warts. The data from 121 patients demonstrated that lidocaine and prilocaine cream was not effective as
a sole anesthetic agent in managing the pain from the surgical procedure. The administration of
lidocaine and prilocaine cream prior to lidocaine infiltration provided significant relief of discomfort
associated with local anesthetic infiltration and thus was effective in the overall reduction of pain from
the procedure only when used in conjunction with local anesthetic infiltration of lidocaine.
Lidocaine and prilocaine cream was studied in 105 full term neonates (gestational age: 37 weeks) for
blood drawing and circumcision procedures. When considering the use of lidocaine and prilocaine
cream in neonates, the primary concerns are the systemic absorption of the active ingredients and the
subsequent formation of methemoglobin. In clinical studies performed in neonates, the plasma levels of
lidocaine, prilocaine, and methemoglobin were not reported in a range expected to cause clinical
symptoms.
Local dermal effects associated with lidocaine and prilocaine cream application in these studies on
intact skin included paleness, redness and edema and were transient in nature (see ADVERSE
REACTIONS).
The application of lidocaine and prilocaine cream on genital mucous membranes for minor, superficial
surgical procedures (eg, removal of condylomata acuminata) was studied in 80 patients in a placebocontrolled
clinical trial (60 patients received lidocaine and prilocaine cream and 20 patients received
placebo). Lidocaine and prilocaine cream (5 to 10 g) applied between 1 and 75 minutes before surgery,
with a median time of 15 minutes, provided effective local anesthesia for minor superficial surgical
procedures. The greatest extent of analgesia, as measured by VAS scores, was attained after 5 to 15
minutes' application. The application of lidocaine and prilocaine cream to genital mucous membranes as
pretreatment for local anesthetic infiltration was studied in a double-blind, placebo-controlled study in
44 female patients (21 patients received lidocaine and prilocaine cream and 23 patients received
placebo) scheduled for infiltration prior to a surgical procedure of the external vulva or genital
mucosa. Lidocaine and prilocaine cream applied to the genital mucous membranes for 5 to 10 minutes
resulted in adequate topical anesthesia for local anesthetic injection.
Individualization of Dose: The dose of lidocaine and prilocaine cream that provides effective
analgesia depends on the duration of the application over the treated area.
All pharmacokinetic and clinical studies employed a thick layer of lidocaine and prilocaine cream (1 to
2 g/10 cm ). The duration of application prior to venipuncture was 1 hour. The duration 2 of application
prior to taking split thickness skin grafts was 2 hours. A thinner application has not been studied and may
result in less complete analgesia or a shorter duration of adequate analgesia.
The systemic absorption of lidocaine and prilocaine is a side effect of the desired local effect. The
amount of drug absorbed depends on surface area and duration of application. The systemic blood
levels depend on the amount absorbed and patient size (weight) and the rate of systemic drug elimination.
Long duration of application, large treatment area, small patients, or impaired elimination may result in
high blood levels. The systemic blood levels are typically a small fraction (1/20 to 1/36) of the blood
levels that produce toxicity. Table 2 below gives maximum recommended doses, application areas and
application times for infants and children.
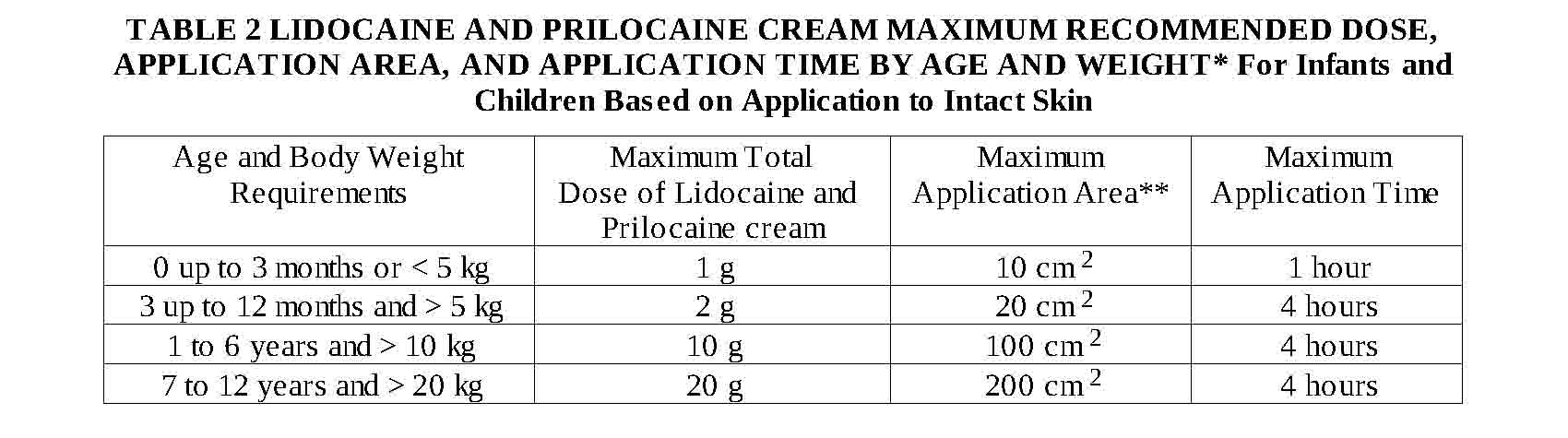
Please note: If a patient greater than 3 months old does not meet the minimum weight requirement, the
maximum total dose of lidocaine and prilocaine cream should be restricted to that which corresponds to
the patient's weight.
* These are broad guidelines for avoiding systemic toxicity in applying lidocaine and prilocaine cream
to patients with normal intact skin and with normal renal and hepatic function.
** For more individualized calculation of how much lidocaine and prilocaine may be absorbed,
physicians can use the following estimates of lidocaine and prilocaine absorption for children and
adults:
The estimated mean (±SD) absorption of lidocaine is 0.045 (±0.016) mg/cm /hr.
The estimated mean (±SD) absorption of prilocaine is 0.077 (±0.036) mg/cm /hr.
An I.V. antiarrhythmic dose of lidocaine is 1 mg/kg (70 mg/70 kg) and gives a blood level of about 1
μg/mL. Toxicity would be expected at blood levels above 5 μg/mL. Smaller areas of treatment are
recommended in a debilitated patient, a small child or a patient with impaired elimination. Decreasing the
duration of application is likely to decrease the analgesic effect.
INDICATIONS & USAGE
Lidocaine and prilocaine cream (a eutectic mixture of lidocaine 2.5% and prilocaine 2.5%) is indicated
as a topical anesthetic for use on:
- normal intact skin for local analgesia.
- genital mucous membranes for superficial minor surgery and as pretreatment for infiltration
anesthesia.
Lidocaine and prilocaine cream is not recommended in any clinical situation when penetration or
migration beyond the tympanic membrane into the middle ear is possible because of the ototoxic effects
observed in animal studies (see WARNINGS).
CONTRAINDICATIONS
Lidocaine and prilocaine cream (lidocaine 2.5% and prilocaine 2.5%) is contraindicated in patients with
a known history of sensitivity to local anesthetics of the amide type or to any other component of the
product.
WARNINGS
Application of lidocaine and prilocaine cream to larger areas or for longer times than those
recommended could result in sufficient absorption of lidocaine and prilocaine resulting in serious
adverse effects (see Individualization of Dose).
Patients treated with class III anti-arrhythmic drugs (e.g., amiodarone, bretylium, sotalol, dofetilide)
should be under close surveillance and ECG monitoring considered, because cardiac effects may be
additive.
Studies in laboratory animals (guinea pigs) have shown that lidocaine and prilocaine cream has an
ototoxic effect when instilled into the middle ear. In these same studies, animals exposed to lidocaine
and prilocaine cream only in the external auditory canal, showed no abnormality. Lidocaine and
prilocaine cream should not be used in any clinical situation when its penetration or migration beyond
the tympanic membrane into the middle ear is possible.
Methemoglobinemia: Lidocaine and prilocaine cream should not be used in those rare patients with
congenital or idiopathic methemoglobinemia and in infants under the age of twelve months who are
receiving treatment with methemoglobin-inducing agents.
Very young patients or patients with glucose-6-phosphate dehydrogenase deficiencies are more
susceptible to methemoglobinemia.
Patients taking drugs associated with drug-induced methemoglobinemia such as sulfonamides,
acetaminophen, acetanilid, aniline dyes, benzocaine, chloroquine, dapsone, naphthalene, nitrates and
nitrites, nitrofurantoin, nitroglycerin, nitroprusside, pamaquine, para-aminosalicylic acid, phenacetin,
phenobarbital, phenytoin, primaquine, quinine, are also at greater risk for developing
methemoglobinemia.
There have been reports of significant methemoglobinemia (20 to 30%) in infants and children
following excessive applications of lidocaine and prilocaine cream. These cases involved the use of
large doses, larger than recommended areas of application, or infants under the age of 3 months who did
not have fully mature enzyme systems. In addition, a few of these cases involved the concomitant
administration of methemoglobin-inducing agents. Most patients recovered spontaneously after removal
of the cream. Treatment with IV methylene blue may be effective if required.
Physicians are cautioned to make sure that parents orother caregivers understand the need for careful
application of lidocaine and prilocaine cream, to ensure that the doses and areas of application
recommended in Table 2 are not exceeded (especially in children under the age of 3 months) and to limit
the period of application to the minimum required to achieve the desired anesthesia.
Neonates and infants up to 3 months of age should be monitored for Met-Hb levels before, during, and
after the application of lidocaine and prilocaine cream, provided the test results can be obtained quickly.
PRECAUTION
General: Repeated doses of lidocaine and prilocaine cream may increase blood levels of lidocaine and
prilocaine. Lidocaine and prilocaine cream should be used with caution in patients who may be more
sensitive to the systemic effects of lidocaine and prilocaine including acutely ill, debilitated, or elderly
patients.
Lidocaine and prilocaine cream should not be applied to open wounds.
Care should be taken not to allow lidocaine and prilocaine cream to come in contact with the eye
because animal studies have demonstrated severe eye irritation. Also the loss of protective reflexes can
permit corneal irritation and potential abrasion. Absorption of lidocaine and prilocaine cream in
conjunctival tissues has not been determined. If eye contact occurs, immediately wash out the eye with
water or saline and protect the eye until sensation returns.
Patients allergic to paraaminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not
shown cross sensitivity to lidocaine and/or prilocaine, however, lidocaine and prilocaine cream should
be used with caution in patients with a history of drug sensitivities, especially if the etiologic agent is
uncertain.
Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally,
are at greater risk of developing toxic plasma concentrations of lidocaine and prilocaine.
Lidocaine and prilocaine have been shown to inhibit viral and bacterial growth. The effect of lidocaine
and prilocaine cream on intradermal injections of live vaccines has not been determined.
Information for Patients : When lidocaine and prilocaine cream is used, the patient should be aware that
the production of dermal analgesia may be accompanied by the block of all sensations in the treated skin.
For this reason, the patient should avoid inadvertent trauma to the treated area by scratching, rubbing, or
exposure to extreme hot or cold temperatures until complete sensation has returned.
Lidocaine and prilocaine cream should not be applied near the eyes or on open wounds.
Drug Interactions : Lidocaine and prilocaine cream should be used with caution in patients receiving
Class I antiarrhythmic drugs (such as tocainide and mexiletine) since the toxic effects are additive and
potentially synergistic.
Prilocaine may contribute to the formation of methemoglobin in patients treated with other drugs
known to cause this condition (see Methemoglobinemia subsection of WARNINGS).
Specific interaction studies with lidocaine/prilocaine and class III anti-arrhythmic drugs (e.g.,
amiodarone, bretylium, sotalol, doetilide) have not been performed, but caution is advised (see
WARNINGS).
Should lidocaine and prilocaine cream be used concomitantly with other products containing lidocaine
and/or prilocaine, cumulative doses from all formulations must be considered.
Carcinogenesis , Mutagenesis , Impairment of Fertility
Carcinogenesis: Long-term studies in animals designed to evaluate the carcinogenic potential of
lidocaine and prilocaine have not been conducted.
Metabolites of prilocaine have been shown to be carcinogenic in laboratory animals. In the animal
studies reported below, doses or blood levels are compared with the Single Dermal Administration
(SDA) of 60 g of lidocaine and prilocaine cream to 400 cm for 3 hours to a small person (50 kg). The
typical application of lidocaine and prilocaine cream for one or two treatments for venipuncture sites
(2.5 or 5 g) would be 1/24 or 1/12 of that dose in an adult or about the same mg/kg dose in an infant.
Chronic oral toxicity studies of ortho-toluidine, a metabolite of prilocaine, in mice (450 to 7200 mg/m
; 60 to 960 times SDA) and rats (900 to 4,800 mg/m ; 60 to 320 times SDA) have shown that orthotoluidine
is a carcinogen in both species. The tumors included hepatocarcinomas/adenomas in female
mice, multiple occurrences of hemangiosarcomas/hemangiomas in both sexes of mice, sarcomas of
multiple organs, transitional-cell carcinomas/papillomas of urinary bladder in both sexes of rats,
subcutaneous fibromas/fibrosarcomas and mesotheliomas in male rats, and mammary gland
fibroadenomas/adenomas in female rats. The lowest dose tested (450 mg/m in mice, 900 mg/m in
rats; 60 times SDA) was carcinogenic in both species. Thus the no-effect dose must be less than 60
times SDA. The animal studies were conducted at 150 to 2,400 mg/kg in mice and at 150 to 800 mg/kg
in rats. The dosages have been converted to mg/m for the SDA calculations above.
Mutagenesis: The mutagenic potential of lidocaine HCl has been tested in a bacterial reverse (Ames)
assay in Salmonella, an in vitro chromosomal aberration assay using human lymphocytes an in vivo
micronucleus test in mice. There was no indication of mutagenicity or structural damage to
chromosomes in these tests.
Ortho-toluidine, a metabolite of prilocaine, at a concentration of 0.5 μg/mL, was genotoxic in
Escherichia coli DNA repair and phage-induction assays. Urine concentrates from rats treated with
ortho-toluidine (300 mg/kg orally; 300 times SDA) were mutagenic when examined in Salmonella
typhimurium in the presence of metabolic activation. Several other tests on ortho-toluidine, including
reverse mutations in five different Salmonella typhimurium strains in the presence or absence of
metabolic activation and a study to detect single strand breaks in DNA of V79 Chinese hamster cells,
were negative.
Impairment of Fertility: See Use in Pregnancy.
Use in Pregnancy: Teratogenic Effects : Pregnancy Category B.
Reproduction studies with lidocaine have been performed in rats and have revealed no evidence of harm
to the fetus (30 mg/kg subcutaneously; 22 times SDA). Reproduction studies with prilocaine have been
performed in rats and have revealed no evidence of impaired fertility or harm to the fetus (300 mg/kg
intramuscularly; 188 times SDA). There are, however, no adequate and well-controlled studies in
pregnant women. Because animal reproduction studies are not always predictive of human response,
lidocaine and prilocaine cream should be used during pregnancy only if clearly needed.
Reproduction studies have been performed in rats receiving subcutaneous administration of an aqueous
mixture containing lidocaine HCl and prilocaine HCl at 1:1 (w/w). At 40 mg/kg each, a dose equivalent
to 29 times SDA lidocaine and 25 times SDA prilocaine, no teratogenic, embryotoxic or fetotoxic
effects were observed.
Labor and Delivery: Neither lidocaine nor prilocaine are contraindicated in labor and delivery. Should
lidocaine and prilocaine cream be used concomitantly with other products containing lidocaine and/or
prilocaine, cumulative doses from all formulations must be considered.
Nursing Mothers : Lidocaine, and probably prilocaine, are excreted in human milk. Therefore, caution
should be exercised when lidocaine and prilocaine cream is administered to a nursing mother since the
milk:plasma ratio of lidocaine is 0.4 and is not determined for prilocaine.
Pediatric Use: Controlled studies of lidocaine and prilocaine cream in children under the age of seven
years have shown less overall benefit than in older children or adults. These results illustrate the
importance of emotional and psychological support of younger children undergoing medical or
surgical procedures.
Lidocaine and prilocaine cream should be used with care in patients with conditions or therapy
associated with methemoglobinemia (see Methemoglobinemia subsection of WARNINGS).
When using lidocaine and prilocaine cream in young children, especially infants under the age of 3
months, care must be taken to insure that the caregiver understands the need to limit the dose and area of
application, and to prevent accidental ingestion (see DOSAGE AND ADMINISTRATION and
Methemoglobinemia).
In neonates (minimum ges tation age: 37 weeks ) and children weighing les s than 20 kg, the area
and duration of application should be limited (see TABLE 2 in Individualization of Dose).
Studies have not demonstrated the efficacy of lidocaine and prilocaine cream for heel lancing in
neonates.
Geriatric Use: Of the total number of patients in clinical studies of lidocaine and prilocaine cream, 180
were age 65 to 74 and 138 were 75 and over. No overall differences in safety or efficacy were
observed between these patients and younger patients. Other reported clinical experience has not
identified differences in responses between the elderly and younger patients, but greater sensitivity of
some older individuals cannot be ruled out.
Plasma levels of lidocaine and prilocaine in geriatric and non-geriatric patients following application of
a thick layer of lidocaine and prilocaine cream are very low and well below potentially toxic levels.
However, there are no sufficient data to evaluate quantitative differences in systemic plasma levels of
lidocaine and prilocaine between geriatric and non-geriatric patients following application of lidocaine
and prilocaine cream.
Consideration should be given for those elderly patients who have enhanced sensitivity to systemic
absorption (see PRECAUTIONS).
After intravenous dosing, the elimination half-life of lidocaine is significantly longer in elderly patients
(2.5 hours) than in younger patients (1.5 hours). (See CLINICAL PHARMACOLOGY).
ADVERSE REACTIONS
Localized Reactions : During or immediately after treatment with lidocaine and prilocaine cream on
intact skin, the skin at the site of treatment may develop erythema or edema or may be the locus of
abnormal sensation. Rare cases of discrete purpuric or petechial reactions at the application site have
been reported. Rare cases of hyperpigmentation following the use of lidocaine and prilocaine cream
have been reported. The relationship to lidocaine and prilocaine cream or the underlying procedure has
not been established. In clinical studies on intact skin involving over 1,300 lidocaine and prilocaine
cream-treated subjects, one or more such local reactions were noted in 56% of patients, and were
generally mild and transient, resolving spontaneously within 1 or 2 hours. There were no serious
reactions that were ascribed to lidocaine and prilocaine cream.
Two recent reports describe blistering on the foreskin in neonates about to undergo circumcision. Both
neonates received 1.0 g of lidocaine and prilocaine cream.
In patients treated with lidocaine and prilocaine cream on intact skin, local effects observed in the trials
included: paleness (pallor or blanching) 37%, redness (erythema) 30%, alterations in temperature
sensations 7%, edema 6%, itching 2% and rash, less than 1%.
In clinical studies on genital mucous membranes involving 378 lidocaine and prilocaine cream-treated
patients, one or more application site reactions, usually mild and transient, were noted in 41% of
patients. The most common application site reactions were redness (21%), burning sensation (17%) and
edema (10%).
Allergic Reactions : Allergic and anaphylactoid reactions associated with lidocaine or prilocaine can
occur. They are characterized by urticaria, angioedema, bronchospasm, and shock. If they occur they
should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful
value.
Systemic (Dose Related) Reactions : Systemic adverse reactions following appropriate use of
lidocaine and prilocaine cream are unlikely due to the small dose absorbed (see Pharmacokinetics
subsection of CLINICAL PHARMACOLOGY). Systemic adverse effects of lidocaine and/or
prilocaine are similar in nature to those observed with other amide local anesthetic agents including
CNS excitation and/or depression (light-headedness, nervousness, apprehension, euphoria, confusion,
dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness,
twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest). Excitatory CNS
reactions may be brief or not occur at all, in which case the first manifestation may be drowsiness
merging into unconsciousness. Cardiovascular manifestations may include bradycardia, hypotension and
cardiovascular collapse leading to arrest.
OVERDOSAGE
Peak blood levels following a 60 g application to 400 cm of intact skin for 3 hours are 0.05 to 0.16
μg/mL for lidocaine and 0.02 to 0.10 μg/mL for prilocaine. Toxic levels of lidocaine (>5 μg/mL) and/or
prilocaine (>6 μg/mL) cause decreases in cardiac output, total peripheral resistance and mean arterial
pressure. These changes may be attributable to direct depressant effects of these local anesthetic agents
on the cardiovascular system. In the absence of massive topical overdose or oral ingestion, evaluation
should include evaluation of other etiologies for the clinical effects or overdosage from other sources
of lidocaine, prilocaine or other local anesthetics. Consult the package inserts for parenteral Xylocaine
(lidocaine HCl) or Citanest (prilocaine HCl) for further information for the management of overdose.
DOSAGE & ADMINISTRATION
Adult Patients -Intact Skin
A thick layer of lidocaine and prilocaine cream is applied to intact skin and covered with an occlusive
dressing (see INSTRUCTIONS FOR APPLICATION).
Minor Dermal Procedures : For minor procedures such as intravenous cannulation and venipuncture,
apply 2.5 grams of lidocaine and prilocaine cream (1/2 the 5 g tube) over 20 to 25 cm of skin surface
for at least 1 hour. In controlled clinical trials using lidocaine and prilocaine cream, two sites were
usually prepared in case there was a technical problem with cannulation or venipuncture at the first site.
Major Dermal Procedures : For more painful dermatological procedures involving a larger skin area
such as split thickness skin graft harvesting, apply 2 grams of lidocaine and prilocaine cream per 10 cm
of skin and allow to remain in contact with the skin for at least 2 hours.
Adult Male Genital Skin: As an adjunct prior to local anesthetic infiltration, apply a thick layer of
lidocaine and prilocaine cream (1 g/10 cm ) to the skin surface for 15 minutes. Local anesthetic
infiltration should be performed immediately after removal of lidocaine and prilocaine cream.
Dermal analgesia can be expected to increase for up to 3 hours under occlusive dressing and persist for
1 to 2 hours after removal of the cream. The amount of lidocaine and prilocaine absorbed during the
period of application can be estimated from the information in Table 2, ** footnote, in Individualization
of Dose.
Adult Female Patients -Genital Mucous Membranes
For minor procedures on the female external genitalia, such as removal of condylomata acuminata, as
well as for use as pretreatment for anesthetic infiltration, apply a thick layer (5 to 10 grams) of lidocaine
and prilocaine cream for 5 to 10 minutes.
Occlusion is not necessary for absorption, but may be helpful to keep the cream in place. Patients
should be lying down during the lidocaine and prilocaine cream application, especially if no occlusion
is used. The procedure or the local anesthetic infiltration should be performed immediately after the
removal of lidocaine and prilocaine cream.
Pediatric Patients -Intact Skin
The following are the maximum recommended doses, application areas and application times for
lidocaine and prilocaine cream based on a child's age and weight:
| Age and Body Weight Requirements |
Maximum Total Prilocaine Cream | Maximum Application Area | Maximum Application Time |
| 0 up to 3 months or < 5 kg | 1g | 10 cm2 | 1 hour |
| 3 up to 12 months and > 5 kg | 2g | 20 cm2 | 4 hours |
| 1 to 6 years and > 10 kg | 10g | 100 cm2 | 4 hours |
| 7 to 12 years and > 20 kg | 20g | 200 cm2 | 4 hours |
Please note: If a patient greater than 3 months old does not meet the minimum weight requirement, the
maximum total dose of lidocaine and prilocaine cream should be restricted to that which corresponds to
the patient's weight (see INSTRUCTIONS FOR APPLICATION).
Practitioners should carefully instruct caregivers to avoid application of excessive amounts of
lidocaine and prilocaine cream (see PRECAUTIONS).
When applying lidocaine and prilocaine cream to the skin of young children, care must be taken to
maintain careful observation of the child to prevent accidental ingestion of lidocaine and prilocaine
cream or the occlusive dressing. A secondary protective covering to prevent inadvertent disruption of
the application site may be useful.
Lidocaine and prilocaine cream s hould not be us ed in neonates with a ges tational age les s than 37
weeks nor in infants under the age of 12 months who are receiving treatment with
methemoglobin-inducing agents (s ee Methemoglobinemia s ubs ection of WARNINGS).
When lidocaine and prilocaine cream (lidocaine 2.5% and prilocaine 2.5%) is used concomitantly with
other products containing local anesthetic agents, the amount absorbed from all formulations must be
considered (see Individualization of Dose). The amount absorbed in the case of lidocaine and prilocaine
cream is determined by the area over which it is applied and the duration of application under occlusion
(see Table 2, ** footnote, in Individualization of Dose).
Although the incidence of systemic adverse reactions with lidocaine and prilocaine cream is very low,
caution should be exercised, particularly when applying it over large areas and leaving it on for longer
than 2 hours. The incidence of systemic adverse reactions can be expected to be directly proportional
to the area and time of exposure (see Individualization of Dose).
INSTRUCTION FOR APPLICATION
To measure 1 gram of lidocaine and prilocaine cream, the Cream should be gently squeezed out of the
tube as a narrow strip that is 1.5 inches (3.8 cm) long and 0.2 inches (5 mm) wide. The strip of lidocaine
and prilocaine cream should be contained within the lines of the diagram shown below.

Use the number of strips that equals your dose, like the examples in the table below.
Dosing Information
1 gram = 1 strip
2 grams = 2 strips
2.5 grams = 2.5 strips
For adult and pediatric patients, apply ONLY as prescribed by your physician.
If your child is below the age of 3 months or small for their age, please inform your doctor before
applying lidocaine and prilocaine cream, which can be harmful, if applied over too much skin at one
time in young children.
When applying lidocaine and prilocaine cream to the intact skin of young children, it is important that
they be carefully observed by an adult in order to prevent the accidental ingestion of or eye contact with
lidocaine and prilocaine cream.
Lidocaine and prilocaine cream must be applied to intact skin at least 1 hour before the start of a routine
procedure and for 2 hours before the start of a painful procedure. A protective covering of the cream is
not necessary for absorption but may be helpful to keep the cream in place.
If using a protective covering, your doctor will remove it, wipe off the lidocaine and prilocaine cream,
and clean the entire area with an antiseptic solution before the procedure. The duration of effective skin
anesthesia will be at least 1 hour after removal of the protective covering.
Precautions
1. Do not apply near eyes or open wounds.
2. Keep out of the reach of children.
3. If your child becomes very dizzy, excessively sleepy, or develops duskiness of the face or lips
after applying lidocaine and prilocaine cream, remove the cream and contact the child's physician at
once.
HOW SUPPLIED
Priloxx LP External Pak (Lidocaine 2.5% and Prilocaine 2.5% Cream, USP) with Occlusive
Dressing Contains:
| Quantity | Size | Description |
|
3 Tubes | 30g/Tube |
packed individually; in a child-resistant tube |
| 20 |
occlusive | dressing packed individually |
NOT FOR OPHTHALMIC USE.
KEEP CONTAINER TIGHTLY CLOSED AT ALL TIMES WHEN NOT IN USE.
Storage: Store at 20º to 25ºC (68º to 77ºF) [see USP Controlled Room Temperature].
Rx only
Keep out of the reach of children.
For all medical inquiries contact:
ACTAVIS
Medical Communications
Parsippany, NJ 07054
1-800-272-5525
Manufactured by:
IGI Laboratories Inc.
Buena, NJ 08310 USA
Priloxx LPTM External Pak
(Lidocaine 2.5% and Prilocaine 2.5% Cream, USP and Frame Style Transparent Dressing)
Packaged for:
Sircle Laboratories, LLC
110 Lexington Dr., Suite E
Madison, MS 39110
For questions or information call toll-free: 1-888-452-9975
visit: www.sirclelabs.com
| PRILOXX LP EXTERNAL KIT
lidocaine and prilocaine cream kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sircle Laboratories LLC (962175621) |
| Registrant - Sircle Laboratories LLC (034356730) |
