NOVA COMPLEX HQ- hydroquinone emulsion
Pharma Cosmetics
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
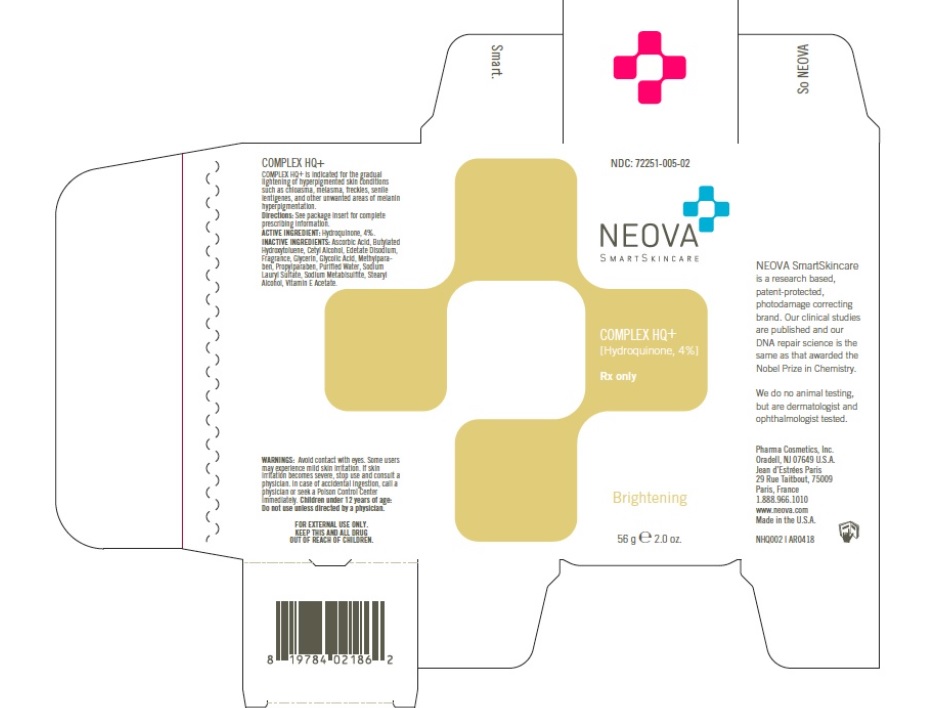
Neova Complex HQ+ (Hydroquinone 4%)
Indications & Usage:
COMPLEX HQ+ is indicated for the gradual lightening of
hyperpigmented skin conditions, such as chloasma, melasma, freckles, senile
lentigines, and other unwanted areas of melanin hyperpigmentation.
Sunscreen must be used daily during COMPLEX HQ+ treatment.
Warnings
- A. Caution: Hydroquinone is a skin bleaching agent which may produce
unwanted cosmetic effects if not used as directed. The physician should be
familiar with the contents of this insert before prescribing or dispensing
this medication. - Test for skin sensitivity before using Hydroquinone Cream by applying asmall amount to an unbroken patch of skin and check in 24 hours. Minorredness is not a contraindication, but where there is itching or vesicleformation or excessive inflammatory response, further treatment is notadvised. Close patient supervision is recommended.Contact with the eyes should be avoided. In case of accidental contact, pa-
tient should rinse eyes thoroughly with water and contact physician. A bitter taste and anesthetic effect may occur if applied to lips. Keep out of reach of children. If no bleaching or lightening effect is noted after 2 months oftreatment use, Hydroquinone Cream should be discontinued. This product isformulated for use as a skin bleaching agent and should not be used for the prevention of sunburn.
-
Sunscreen use is an essential aspect of Hydroquinone therapy becauseeven minimal sunlight exposure sustains melanocytic activity. After clear-ing and during maintenance therapy, sun exposure should be avoided on bleached skin by application of a sunscreen or sunblock agent, or protective clothing to prevent repigmentation.
-
There are no sunblocking or sunscreening agents in COMPLEX HQ+ and since minimal sunlight exposure may reverse the bleaching effect of this preparation, it should be used only at night or on areas of the body covered by protective clothing. During the daytime, sunblocking or broad spectrum sunscreen preparations or protective clothing should be used to prevent the
bleached areas from repigmentation. -
Keep this and all medications out of the reach of children. In case of ac-cidental ingestion, call a physician or a poison control center immediately.
-
Warning: Contains sodium metabisulfite, a sulfite that may cause serious allergic type reactions (e.g. hives, itching, wheezing, anaphylaxis, severe asthma attacks) in certain susceptible persons.
Drug Dosage and Administration:
Apply a thin layer on the skin surface in the affected areas twice a day or as directed by physician. Use of this productshould be discontinued after 3 months of treatment if no improvement is observed. The lightening effect may not be noticeable when used on very dark skin.Sun exposure should be avoided. DNA Damage ControlTM [EVERYDAY | BROAD SPECTRUM SPF 44] or other broad spectrum sunscreen or protective clothing should be used to prevent reoccurring hyperpigmentation.
| NOVA COMPLEX HQ
hydroquinone emulsion |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Pharma Cosmetics (080622701) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanitor Corporation | 797472792 | manufacture(72251-005) | |